Treating to target in multiple sclerosis: Do we know how to measure whether we hit it?
- PMID: 39445673
- PMCID: PMC11554867
- DOI: 10.1111/ene.16526
Treating to target in multiple sclerosis: Do we know how to measure whether we hit it?
Abstract
Background and purpose: The rapidly evolving landscape of effective treatment options in multiple sclerosis has led to a shift of treatment objectives towards a treat-to-target approach aiming to suppress disease activity below the level of detectability early during the disease. To enable treat-to-target, a thorough reappraisal of available outcome measures with respect to their ability in this regard is required.
Methods: To that end, we conducted a comprehensive systematic literature review of more than 1000 studies using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 methodology focusing on underlying evidence as well as utility and implementability in clinical practice.
Results: From there, we propose a set of measurable outcomes for everyday routine clinical practice as well as advanced/aspirational measurables requiring additional resources. We also outline remaining knowledge/technology gaps that need to be overcome to enable a treat-to-target approach.
Conclusions: This work provides the basis for an evidence-based definition of outcome targets for relevant stakeholders and regulatory authorities.
Keywords: applicability; measures; multiple sclerosis; outcome; review; treat‐to‐target; utility.
© 2024 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
G.B. has participated in meetings sponsored by or received speaker honoraria or travel funding from Biogen, Celgene/BMS, Janssen, Lilly, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva and has received honoraria for consulting from Biogen, Celgene/BMS, Janssen, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis. N.K. has participated in meetings sponsored by or received speaker honoraria or travel funding from Alexion, BMS/Celgene, Janssen‐Cilag, Merck, Novartis, Roche, and Sanofi‐Genzyme and has held a grant for a Multiple Sclerosis Clinical Training Fellowship Program from the European Committee for Treatment and Research in Multiple Sclerosis. P.A. has participated in meetings sponsored by or received speaker honoraria or travel funding from Biogen, Merck, Roche, Sanofi‐Genzyme, and Teva and has received honoraria for consulting from Biogen. He received a research grant from Quanterix International and was awarded a combined sponsorship from Biogen, Merck, Sanofi‐Genzyme, Roche, and Teva for a clinical study. B.H. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from Novartis, Genentech, Biogen, Sanofi, EMD Serono, Amgen, Bristol, Myers, Squibb, and Alexion. T.Bh. has received honoraria and consulting fees from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Envision Pharma, Flatiron UK, Heel Pharma, Hollister, Insmed, King's College London, Medable, Medidata, Merck, MSD, Novartis, Oxford Health, Parexel, Pfizer, Queen Mary University London, Roche, Sandoz, Servier, Talking Medicines, Teva, UCB, University College London, and University of Nottingham. S.J. has participated in meetings sponsored by Merck, Roche, Sanofi‐Genzyme, and Teva and has received honoraria for consulting from Novartis. F.L. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from Biogen, EMD Serono, Novartis, Actelion/Janssen, Sanofi‐Genzyme, Roche/Genentech, Horizon Therapeutics/Amgen, Celgene/BMS, Mapi Pharma, Brainstorm Cell Therapeutics, Mylan/Viatris, Immunic, Avotres, Neurogene, LabCorp, Entelexo Biotherapeutics, Neuralight, SetPoint Medical, Hexal/Sandoz, Baim Institute, Sudo Biosciences, Lapix Therapeutics, Biohaven Pharmaceuticals, Abata Therapeutics, Cognito Therapeutics, and ImmPACT Bio. His institution has received financial support for research from Biogen, Novartis, Sanofi, NMSS, NIH, and Brainstorm Cell Therapeutics. J.O. has received grant funding from Biogen‐Idec, Roche, and EMD‐Serono and has received personal compensation for consulting or speaking from Biogen‐Idec, BMS, EMD‐Serono, Eli Lilly, Horizon Therapeutics, Novartis, Roche, and Sanofi‐Genzyme. D.P. has received honoraria for consulting and advising from Celgene/BMS, Janssen, and Novartis. A.R. has been an employee of icometrix (a company aiming to bring relevant outcome measures to clinical practice). A.S. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS including Biogen, Merck, Mylan, Novartis, Roche, Sanofi‐Genzyme, and Viatris. D.S. has been an employee of icometrix (a company aiming to bring relevant outcome measures to clinical practice). E.T. has received consulting and lecturing fees, travel grants, or unconditional research support from the following pharmaceutical companies: Actelion, Biogen, BMS, Merck, Novartis, Roche, and Teva Pharma. A.C. has received speaker/board honoraria from Actelion (Janssen/J&J), Alexion, Almirall, Bayer, Biogen, Celgene (BMS), Genzyme, Merck, Novartis, Roche, and Teva, all for hospital research funds. He has received research support from Biogen, CSL Behring, Genzyme, UCB, the European Union, and the Swiss National Foundation. T.Be. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS including Allergan, Bayer, Biogen, Bionorica, BMS/Celgene, Genesis, GSK, GW/Jazz Pharma, Horizon, Janssen‐Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi‐Genzyme, Teva, and UCB. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Roche, Sanofi‐Genzyme, Teva) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, and Teva.
Figures
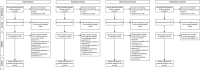




References
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221‐1231. - PubMed
-
- Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3‐18. - PubMed
-
- Yeung J, Bourcier M, Gooderham MJ, et al. Management of moderate‐to‐severe plaque psoriasis with biologics: a treat‐to‐target position paper. Dermatol Ther. 2022;35:e15777. - PubMed
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis. Neurology. 1996;46:907‐911. - PubMed
-
- Bielekova B, Kadom N, Fisher E, et al. MRI as a marker for disease heterogeneity in multiple sclerosis. Neurology. 2005;65:1071‐1076. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

