Mechanisms of Staphylococcus aureus survival of trimethoprim-sulfamethoxazole-induced thymineless death
- PMID: 39445807
- PMCID: PMC11559000
- DOI: 10.1128/mbio.01634-24
Mechanisms of Staphylococcus aureus survival of trimethoprim-sulfamethoxazole-induced thymineless death
Abstract
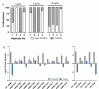
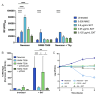
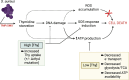
Trimethoprim-sulfamethoxazole (SXT) is commonly used to treat diverse Staphylococcus aureus infections, including those associated with cystic fibrosis (CF) pulmonary disease. Studies with Escherichia coli found that SXT impairs tetrahydrofolate production, leading to DNA damage, stress response induction, and accumulation of reactive oxygen species (ROS) in a process known as thymineless death (TLD). TLD survival can occur through the uptake of exogenous thymidine, countering the effects of SXT; however, a growing body of research has implicated central metabolism as another potentially important determinant of bacterial survival of SXT and other antibiotics. Here, we conducted studies to better understand the mechanisms of TLD survival in S. aureus. We found that thymidine abundances in CF sputum were insufficient to prevent TLD of S. aureus, highlighting the importance of alternative survival mechanisms in vivo. In S. aureus cultured in vitro with SXT and low thymidine, we frequently identified adaptive mutations in genes encoding carbohydrate, nucleotide, and amino acid metabolism, supporting reduced metabolism as a common survival mechanism. Although intracellular ROS levels rose with SXT treatment in vitro, survival was not improved in the presence of ROS scavengers, unlike in E. coli. SXT challenge induced the SOS response, which was alleviated by added thymidine. Finally, an inactivating mutation in the phosphotransferase gene ptsI conferred both limitation in cellular ATP and improved survival against TLD. Collectively, these results suggest that alterations in core metabolic functions, particularly those that reduce ATP levels, predominantly confer S. aureus survival and persistence during SXT treatment, potentially identifying novel targets for co-treatment.IMPORTANCEStaphylococcus aureus is a ubiquitous organism and one of the leading causes of human infections, many of which are difficult to treat due to persistence, antibiotic resistance, or antibiotic tolerance. As our arsenal of effective antibiotics dwindles, the need for improved treatments becomes increasingly urgent, necessitating a better understanding of the precise mechanisms by which pathogens evade our most critical antimicrobial agents. Here, we report a systematic characterization of the mechanisms of S. aureus survival to treatment with the first-line antistaphylococcal antibiotic trimethoprim-sulfamethoxazole, identifying pathways and candidate targets for enhancing the efficacy of available antimicrobial agents.
Keywords: Staphylococcus aureus; antibiotic resistance; antifolate drugs; persistence; thymineless death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- CDC . 2019. Staph infections can kill. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/vitalsigns/staph/index.html
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

