MAIT cells: Conserved watchers on the wall
- PMID: 39446132
- PMCID: PMC11514058
- DOI: 10.1084/jem.20232298
MAIT cells: Conserved watchers on the wall
Abstract
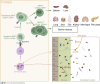
MAIT cells are innate-like T cells residing in barrier tissues such as the lung, skin, and intestine. Both the semi-invariant T cell receptor of MAIT cells and the restricting element MR1 are deeply conserved across mammals, indicating non-redundant functions linked to antigenic specificity. MAIT cells across species concomitantly express cytotoxicity and tissue-repair genes, suggesting versatile functions. Accordingly, MAIT cells contribute to antibacterial responses as well as to the repair of damaged barrier tissues. MAIT cells recognize riboflavin biosynthetic pathway-derived metabolites, which rapidly cross epithelial barriers to be presented by antigen-presenting cells. Changes in gut ecology during intestinal inflammation drive the expansion of strong riboflavin and MAIT ligand producers. Thus, MAIT cells may enable real-time surveillance of microbiota dysbiosis across intact epithelia and provide rapid and context-dependent responses. Here, we discuss recent findings regarding the origin and regulation of MAIT ligands and the role of MAIT cells in barrier tissues. We speculate on the potential reasons for MAIT cell conservation during evolution.
© 2024 Germain et al.
Conflict of interest statement
Disclosures: O. Lantz reported grants and personal fees from Biomunex during the conduct of the study and grants and personal fees from Biomunex outside the submitted work; in addition, O. Lantz had a patent to Car-T MAIT pending. No other disclosures were reported.
Figures

References
-
- Becker, J.M., Kauffman S.J., Hauser M., Huang L., Lin M., Sillaots S., Jiang B., Xu D., and Roemer T.. 2010. Pathway analysis of Candida albicans survival and virulence determinants in a murine infection model. Proc. Natl. Acad. Sci. USA. 107:22044–22049. 10.1073/pnas.1009845107 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

