Atrial cardiomyopathy resulting from loss of plakophilin-2 expression: Response to adrenergic stimulation and implications for the exercise response
- PMID: 39446303
- PMCID: PMC12018593
- DOI: 10.1113/JP286985
Atrial cardiomyopathy resulting from loss of plakophilin-2 expression: Response to adrenergic stimulation and implications for the exercise response
Abstract
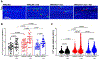
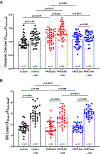
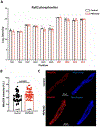
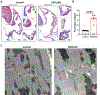
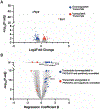
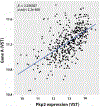
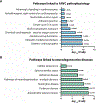
Atrial arrhythmias occur in 20-40% of patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) and are associated with an increased risk of sustained ventricular arrhythmias and inappropriate implantable cardioverter-defibrillator shocks. The pathophysiology of atrial arrhythmias in ARVC remains unclear. Most cases of gene-positive ARVC are linked to pathogenic variants in the desmosomal gene plakophilin-2 (PKP2). Here, we test the hypothesis that loss of PKP2 expression leads to pro-arrhythmic changes in atrial cardiomyocytes. Atrial cells/tissue were obtained from a cardiac-specific, tamoxifen-activated model of PKP2 deficiency (PKP2cKO). By contrast to PKP2cKO ventricular myocytes, PKP2cKO atrial cardiomyocytes presented no significant differences in intracellular calcium (Ca2+ i) transient dynamics, sarcoplasmic reticulum load or action potential morphology. PKP2cKO atrial cardiomyocytes showed elevated reactive oxygen species levels, increased frequency and amplitude of Ca2+ sparks, and increased diastolic [Ca2+]i compared to control; the latter two parameters were further increased by isoproterenol exposure and reversed by exposure to ryanodine receptor blocker dantrolene. We speculate that these isoproterenol-dependent effects may impact on the exercise-related atrial arrhythmia risk in ARVC patients. Despite absence of changes in Ca2+ i transient dynamics, PKP2cKO atrial cardiomyocytes showed enhanced sarcomere shortening and impaired sarcomere relaxation. Orthogonal transcriptomic analysis of human(GTEx) and PKP2cKO atrial tissue led to identification of 41 transcripts depending on PKP2 expression. Biochemical follow-up confirmed reduced abundance of sarcomeric protein myosin binding protein C, potentially playing a role in cellular shortening and relaxation changes observed. Our findings provide novel insights into the role of PKP2 in atrial myocardium with potential implications to therapeutic management of atrial fibrillation in patients with PKP2-related ARVC. KEY POINTS: Atrial arrhythmias occur in a large group of patients with arrhythmogenic right ventricular cardiomyopathy (ARVC), a cardiac disease mostly caused by pathogenic variants in the desmosomal gene plakophilin-2 (PKP2). Exercise is considered to be an independent risk factor for arrhythmias consequent to PKP2 deficiency. We show that loss of PKP2 expression affects cellular calcium handling and electrophysiology differently in left atrial vs. ventricular myocardium and causes extensive atrial fibrosis. PKP2-deficient atrial cardiomyocytes present increased spontaneous sarcoplasmic reticulum calcium release events, further enhanced by isoproterenol exposure and reversible by a ryanodine receptor blocker (dantrolene). In addition, PKP2-deficient atrial myocytes exhibit impaired relaxation and enhanced sarcomere shortening, most probably related to reduced abundance of myosin binding protein C. We speculate that cellular effects reported upon isoproterenol impact on the exercise-related atrial arrhythmia risk in ARVC patients. We further propose that therapeutic approaches aimed at mitigating ventricular damage may be effective to treat the atrial disease in ARVC.
Keywords: adrenergic stimulation; arrhythmogenic right ventricular cardiomyopathy; atrial fibrillation; oxidative stress; plakophilin‐2; ryanodine receptor.
© 2024 The Authors. The Journal of Physiology © 2024 The Physiological Society.
Figures


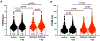



References
-
- Assis FR, Krishnan A, Zhou X, James CA, Murray B, Tichnell C, Berger R, Calkins H, Tandri H & Mandal K (2019). Cardiac sympathectomy for refractory ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 16, 1003–1010. - PubMed
-
- Baturova MA, Haugaa KH, Jensen HK, Svensson A, Gilljam T, Bundgaard H, Madsen T, Hansen J, Chivulescu M, Christiansen MK, Carlson J, Edvardsen T, Svendsen JH & Platonov PG (2020). Atrial fibrillation as a clinical characteristic of arrhythmogenic right ventricular cardiomyopathy: Experience from the Nordic ARVC Registry. Int J Cardiol 298, 39–43. - PubMed
-
- Bhonsale A et al. (2015). Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 36, 847–855. - PubMed
-
- Bootman MD, Higazi DR, Coombes S & Roderick HL (2006). Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. J Cell Sci 119, 3915–3925. - PubMed
-
- Camm CF, James CA, Tichnell C, Murray B, Bhonsale A, te Riele ASJM, Judge DP, Tandri H & Calkins H (2013). Prevalence of atrial arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm 10, 1661–1668. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
