Targeted Deletion of Fibroblast Growth Factor 23 Rescues Metabolic Dysregulation of Diet-induced Obesity in Female Mice
- PMID: 39446375
- PMCID: PMC11538792
- DOI: 10.1210/endocr/bqae141
Targeted Deletion of Fibroblast Growth Factor 23 Rescues Metabolic Dysregulation of Diet-induced Obesity in Female Mice
Abstract
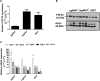
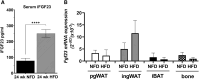
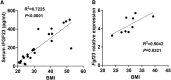
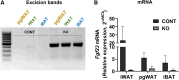
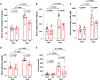
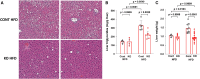
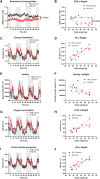
Fibroblast growth factor 23 (FGF23) is a bone-secreted protein widely recognized as a critical regulator of skeletal and mineral metabolism. However, little is known about the nonskeletal production of FGF23 and its role in tissues other than bone. Growing evidence indicates that circulating FGF23 levels rise with a high-fat diet (HFD) and they are positively correlated with body mass index (BMI) in humans. In the present study, we show for the first time that increased circulating FGF23 levels in obese humans correlate with increased expression of adipose Fgf23 and both positively correlate with BMI. To understand the role of adipose-derived Fgf23, we generated adipocyte-specific Fgf23 knockout mice (AdipoqFgf23Δfl/Δfl) using the adiponectin-Cre driver, which targets mature white, beige, and brown adipocytes. Our data show that targeted ablation of Fgf23 in adipocytes prevents HFD-fed female mice from gaining body weight and fat mass while preserving lean mass but has no effect on male mice, indicating the presence of sexual dimorphism. These effects are observed in the absence of changes in food and energy intake. Adipose Fgf23 inactivation also prevents dyslipidemia, hyperglycemia, and hepatic steatosis in female mice. Moreover, these changes are associated with decreased respiratory exchange ratio and increased brown fat Ucp1 expression in knockout mice compared to HFD-fed control mice (Fgf23fl/fl). In conclusion, this is the first study highlighting that targeted inactivation of Fgf23 is a promising therapeutic strategy for weight loss and lean mass preservation in humans.
Keywords: FGF23; adipose tissue; high-fat diet; lipid metabolism; obesity.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures




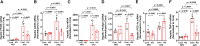

References
-
- Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205‐216. - PubMed
-
- Jastreboff AM, Kaplan LM, Frias JP, et al. Triple-hormone-receptor agonist retatrutide for obesity—a phase 2 trial. N Engl J Med. 2023;389(6):514‐526. - PubMed
-
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989‐1002. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

