Anti-Programmed Death Ligand 1 Plus Targeted Therapy in Anaplastic Thyroid Carcinoma: A Nonrandomized Clinical Trial
- PMID: 39446377
- PMCID: PMC11581602
- DOI: 10.1001/jamaoncol.2024.4729
Anti-Programmed Death Ligand 1 Plus Targeted Therapy in Anaplastic Thyroid Carcinoma: A Nonrandomized Clinical Trial
Abstract
Importance: Anaplastic thyroid carcinoma (ATC) is a rare and lethal cancer. Although progress has been made in recent years in patients with mutated BRAF tumors, those who respond initially eventually die of their disease; furthermore, there are no approved therapies for non-BRAF mutated tumors.
Objective: To determine whether treatment with matched-targeted therapy plus immune checkpoint inhibitors were associated with improved overall survival (OS).
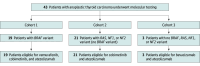
Design, setting, and participants: A phase 2 trial at a single center, tertiary institution with parallel cohorts, assigning treatment with targeted therapy according to the tumor mutation status. Patients with mutated BRAF V600E tumors received vemurafenib/cobimetinib plus atezolizumab (cohort 1); those with mutated RAS (NRAS, KRAS, or HRAS) or NF1/2 tumors received cobimetinib plus atezolizumab (cohort 2). Patients without any of these variants were assigned to receive bevacizumab plus atezolizumab (cohort 3). Patients were enrolled from August 3, 2017, to July 7, 2021. All consecutive, systemic therapy-naive patients with ATC with active disease and who met eligibility criteria were considered for participation. The analysis was conducted in September 2023.
Interventions: Patients were assigned to targeted therapy based on the driver mutation as follow: BRAF V600E (cohort 1, vemurafenib plus cobimetinib), RAS/NF (cohort 2, cobimetinib), or non-BRAF/RAS/NF (cohort 3, bevacizumab). All received atezolizumab.
Main outcomes and measures: The primary outcome of the study was median OS of the entire targeted therapy cohort, compared with historical median OS of 5 months.
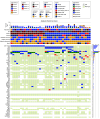
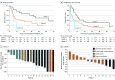
Results: Forty-three patients with ATC were enrolled in the targeted therapy cohorts, of which 42 were included in the primary analysis. The median OS in patients across these 3 cohorts was 19 months (95% CI, 7.79-43.24). Median OS and progression-free survival per cohort were as follows: cohort 1: 43 months (95% CI, 16-not estimable [NE]), 13.9 months (6.6-64.1); cohort 2: 8.7 months (95% CI, 5.1-37.0) and 4.8 months (1.8-14.7); cohort 3 (vascular endothelial growth factor inhibitor group): 6.21 months (4.1-NE) and 1.3 months (1.3-NE), respectively.
Conclusions and relevance: In this nonrandomized clinical trial, atezolizumab combined with targeted therapy resulted in a longer median OS than historical landmark, achieving the study's primary end point, with cohort 1 achieving the longest OS.
Trial registration: ClinicalTrials.gov Identifier: NCT03181100.
Conflict of interest statement
Figures
Comment in
-
Impacts of Immunotherapy on Patients With Aggressive Thyroid Carcinomas.JAMA Oncol. 2024 Dec 1;10(12):1617-1618. doi: 10.1001/jamaoncol.2024.4202. JAMA Oncol. 2024. PMID: 39446352 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

