Metabolic strategies in hypoxic plants
- PMID: 39446413
- PMCID: PMC11663712
- DOI: 10.1093/plphys/kiae564
Metabolic strategies in hypoxic plants
Abstract
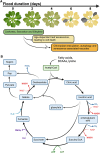
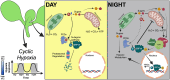
Complex multicellular organisms have evolved in an oxygen-enriched atmosphere. Oxygen is therefore essential for all aerobic organisms, including plants, for energy production through cellular respiration. However, plants can experience hypoxia following extreme flooding events and also under aerated conditions in proliferative organs or tissues characterized by high oxygen consumption. When oxygen availability is compromised, plants adopt different strategies to cope with hypoxia and limited aeration. A common feature among different plant species is the activation of an anaerobic fermentative metabolism to provide ATP to maintain cellular homeostasis under hypoxia. Fermentation also requires many sugar substrates, which is not always feasible, and alternative metabolic strategies are thus needed. Recent findings have also shown that the hypoxic metabolism is also active in specific organs or tissues of the plant under aerated conditions. Here, we describe the regulatory mechanisms that control the metabolic strategies of plants and how they enable them to thrive despite challenging conditions. A comprehensive mechanistic understanding of the genetic and physiological components underlying hypoxic metabolism should help to provide opportunities to improve plant resilience under the current climate change scenario.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest. None declared.
Figures


Similar articles
-
Plant oxygen sensing is mediated by the N-end rule pathway: a milestone in plant anaerobiosis.Plant Cell. 2011 Dec;23(12):4173-83. doi: 10.1105/tpc.111.093880. Epub 2011 Dec 29. Plant Cell. 2011. PMID: 22207573 Free PMC article.
-
Advances in plant oxygen sensing: endogenous and exogenous mechanisms.J Genet Genomics. 2025 May;52(5):615-627. doi: 10.1016/j.jgg.2024.11.014. Epub 2024 Dec 9. J Genet Genomics. 2025. PMID: 39638088 Review.
-
Redox homeostasis in plants. The challenge of living with endogenous oxygen production.Respir Physiol Neurobiol. 2010 Aug 31;173 Suppl:S13-9. doi: 10.1016/j.resp.2010.02.007. Epub 2010 Feb 24. Respir Physiol Neurobiol. 2010. PMID: 20188218 Review.
-
Synthetic biology of hypoxia.New Phytol. 2021 Jan;229(1):50-56. doi: 10.1111/nph.16441. Epub 2020 Apr 1. New Phytol. 2021. PMID: 31960974 Free PMC article. Review.
-
Functional electron microscopy in studies of plant response and adaptation to anaerobic stress.Ann Bot. 2003 Jan;91 Spec No(2):155-72. doi: 10.1093/aob/mcf244. Ann Bot. 2003. PMID: 12509337 Free PMC article. Review.
Cited by
-
Hypoxia as challenge and opportunity: From cells to crops, to synthetic biology.Plant Physiol. 2024 Dec 24;197(1):kiae640. doi: 10.1093/plphys/kiae640. Plant Physiol. 2024. PMID: 39657271 Free PMC article. No abstract available.
-
Involvement of miR775 in the Post-Transcriptional Regulation of Fructose-1,6-Bisphosphate Aldolase in Maize (Zea mays L.) Leaves Under Hypoxia.Int J Mol Sci. 2025 Jan 21;26(3):865. doi: 10.3390/ijms26030865. Int J Mol Sci. 2025. PMID: 39940636 Free PMC article.
-
Decoding plant responses to waterlogging: from stress signals to molecular mechanisms and their future implications.Plant Mol Biol. 2025 Jun 29;115(4):78. doi: 10.1007/s11103-025-01611-8. Plant Mol Biol. 2025. PMID: 40581894 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

