Comparison of postoperative cyclosporine 2.0% versus betamethasone 0.1% eye drops following trabeculectomy: A randomized clinical trial
- PMID: 39446816
- PMCID: PMC12013290
- DOI: 10.4103/IJO.IJO_345_24
Comparison of postoperative cyclosporine 2.0% versus betamethasone 0.1% eye drops following trabeculectomy: A randomized clinical trial
Abstract
Purpose: To investigate the effect of cyclosporine A 2% eye drop following trabeculectomy on intraocular pressure (IOP) and surgical success compared to postoperative steroid drop.
Study design: Prospective, randomized clinical trial in an institutional setting.
Methods patients: Forty patients with primary open-angle glaucoma and candidates for trabeculectomy were included in this study. Standard fornix-based trabeculectomy augmented with mitomycin C was performed for all patients. None of the included subjects had a history of prior laser or intraocular surgery in that eye.
Intervention: All included subjects were randomly assigned to either postoperative cyclosporine A 2% or betamethasone 0.1% eye drops.
Main outcome measures: IOP measured by applanation tonometer and surgical success rate.
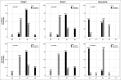
Results: Seventy-five potentially eligible POAG patients were seen during the study period, and 40 met the study criteria and were randomly assigned to one of the study groups. Patients in the cyclosporine A 2% group had consistently lower IOP, fewer glaucoma medications, and higher success rates for at least 24 months after surgery ( P < 0.0001). Complete success was more frequent in the cyclosporine group. Moreover, the cyclosporine A group had more diffuse and elevated bleb with less vascularity in the first 3 months after surgery ( P ≤ 0.01). This was paralleled with fewer dry eye signs and symptoms in the cyclosporine group in the first 3 months ( P < 0.03).
Conclusion: Postoperative cyclosporine A 2% eye drop can be used instead of steroid drops and is associated with improved surgical success and decreased dry eye manifestations.
Copyright © 2024 Indian Journal of Ophthalmology.
Conflict of interest statement
There are no conflicts of interest.
Figures






References
-
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. - PubMed
-
- Mansouri K, Tanna AP, De Moraes CG, Camp AS, Weinreb RN. Review of the measurement and management of 24-hour intraocular pressure in patients with glaucoma. Surv Ophthalmol. 2020;65:171–86. - PubMed
-
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130:429–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

