L-Arginine and asymmetric dimethylarginine (ADMA) transport across the mouse blood-brain and blood-CSF barriers: Evidence of saturable transport at both interfaces and CNS to blood efflux
- PMID: 39446890
- PMCID: PMC11501026
- DOI: 10.1371/journal.pone.0305318
L-Arginine and asymmetric dimethylarginine (ADMA) transport across the mouse blood-brain and blood-CSF barriers: Evidence of saturable transport at both interfaces and CNS to blood efflux
Abstract
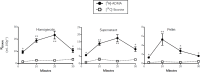
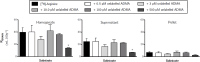
L-Arginine is the physiological substrate for the nitric oxide synthase (NOS) family, which synthesises nitric oxide (NO) in endothelial and neuronal cells. NO synthesis can be inhibited by endogenous asymmetric dimethylarginine (ADMA). NO has explicit roles in cellular signalling and vasodilation. Impaired NO bioavailability represents the central feature of endothelial dysfunction associated with vascular diseases. Interestingly, dietary supplementation with L-arginine has been shown to alleviate endothelial dysfunctions caused by impaired NO synthesis. In this study the transport kinetics of [3H]-arginine and [3H]-ADMA into the central nervous system (CNS) were investigated using physicochemical assessment and the in situ brain/choroid plexus perfusion technique in anesthetized mice. Results indicated that L-arginine and ADMA are tripolar cationic amino acids and have a gross charge at pH 7.4 of 0.981. L-Arginine (0.00149±0.00016) has a lower lipophilicity than ADMA (0.00226±0.00006) as measured using octanol-saline partition coefficients. The in situ perfusion studies revealed that [3H]-arginine and [3H]-ADMA can cross the blood-brain barrier (BBB) and the blood-CSF barrier. [3H]-Arginine (11.6nM) and [3H]-ADMA (62.5nM) having unidirectional transfer constants (Kin) into the frontal cortex of 5.84±0.86 and 2.49±0.35 μl.min-1.g-1, respectively, and into the CSF of 1.08±0.24 and 2.70±0.90 μl.min-1.g-1, respectively. In addition, multiple-time uptake studies revealed the presence of CNS-to-blood efflux of ADMA. Self- and cross-inhibition studies indicated the presence of transporters at the BBB and the blood-CSF barriers for both amino acids, which were shared to some degree. Importantly, these results are the first to demonstrate: (i) saturable transport of [3H]-ADMA at the blood-CSF barrier (choroid plexus) and (ii) a significant CNS to blood efflux of [3H]-ADMA. Our results suggest that the arginine paradox, in other words the clinical observation that NO-deficient patients respond well to oral supplementation with L-arginine even though the plasma concentration is sufficient to saturate endothelial NOS, could be related to altered ADMA transport (efflux).
Copyright: © 2024 Fidanboylu, Thomas. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




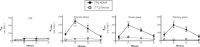




Similar articles
-
Saturation kinetics and specificity of transporters for L-arginine and asymmetric dimethylarginine (ADMA) at the blood-brain and blood-CSF barriers.PLoS One. 2025 Jun 11;20(6):e0320034. doi: 10.1371/journal.pone.0320034. eCollection 2025. PLoS One. 2025. PMID: 40498714 Free PMC article.
-
The transporter and permeability interactions of asymmetric dimethylarginine (ADMA) and L-arginine with the human blood-brain barrier in vitro.Brain Res. 2016 Oct 1;1648(Pt A):232-242. doi: 10.1016/j.brainres.2016.07.026. Epub 2016 Jul 16. Brain Res. 2016. PMID: 27431938 Free PMC article.
-
Ammonia Reduces Intracellular Asymmetric Dimethylarginine in Cultured Astrocytes Stimulating Its y⁺LAT2 Carrier-Mediated Loss.Int J Mol Sci. 2017 Nov 2;18(11):2308. doi: 10.3390/ijms18112308. Int J Mol Sci. 2017. PMID: 29099056 Free PMC article.
-
Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the "L-arginine paradox" and acts as a novel cardiovascular risk factor.J Nutr. 2004 Oct;134(10 Suppl):2842S-2847S; discussion 2853S. doi: 10.1093/jn/134.10.2842S. J Nutr. 2004. PMID: 15465797 Review.
-
Asymmetric dimethylarginine (ADMA) and other endogenous nitric oxide synthase (NOS) inhibitors as an important cause of vascular insulin resistance.Horm Metab Res. 2008 Sep;40(9):655-9. doi: 10.1055/s-0028-1083814. Epub 2008 Sep 15. Horm Metab Res. 2008. PMID: 18792879 Review.
Cited by
-
Saturation kinetics and specificity of transporters for L-arginine and asymmetric dimethylarginine (ADMA) at the blood-brain and blood-CSF barriers.PLoS One. 2025 Jun 11;20(6):e0320034. doi: 10.1371/journal.pone.0320034. eCollection 2025. PLoS One. 2025. PMID: 40498714 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

