Evaluation of Effective Half-Life and Its Impact on Time to Steady State for Oral MeltDose Tacrolimus (LCPT) in De Novo Kidney Transplant Recipients
- PMID: 39446891
- PMCID: PMC11702898
- DOI: 10.1097/FTD.0000000000001270
Evaluation of Effective Half-Life and Its Impact on Time to Steady State for Oral MeltDose Tacrolimus (LCPT) in De Novo Kidney Transplant Recipients
Abstract
Background: For extended-release drug formulations, effective half-life (t 1/2eff ) is a relevant pharmacokinetic parameter to inform dosing strategies and time to reach steady state. Tacrolimus, an immunosuppressant commonly used for the prophylaxis of organ rejection in transplant patients, is available as both immediate- and extended-release formulations. To the best of our knowledge, the t 1/2eff of tacrolimus from these different formulations has not yet been assessed. The objective of this study was to characterize the t 1/2eff and terminal half-life (t 1/2z ) of an extended-release once-daily tacrolimus formulation (LCPT) and twice-daily immediate-release tacrolimus (IR-Tac).
Methods: A noncompartmental analysis of pharmacokinetic data obtained from a phase 2 study in de novo kidney transplant recipients receiving either LCPT or IR-Tac was conducted. Intensive blood sampling was performed on days 1, 7, and 14, and tacrolimus whole blood concentrations were measured using a validated liquid chromatography with tandem mass spectrometry method. T 1/2eff was estimated using within-participant accumulation ratios. T 1/2z was estimated by linear regression of the terminal phase of the concentration versus time profile.
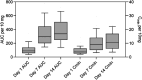
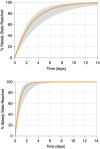
Results: The median accumulation ratios of LCPT and IR-Tac on day 14 were 3.18 and 2.06, respectively.The median (interquartile range; IQR) t 1/2eff for LCPT at day 14 of dosing was 48.4 (37.4-77.9) hours, whereas the t 1/2z was 20.3 (17.6-22.9) hours. For IR-Tac, the median (IQR) t 1/2eff and t 1/2z on day 14 were 12.5 (8.8-23.0) hours and 12.2 (9.2-15.7) hours, respectively.
Conclusions: Consistent with its prolonged release of tacrolimus, LCPT demonstrated a higher accumulation ratio and a longer t 1/2eff compared with IR-Tac. These findings underscore the pharmacokinetic differences between different drug formulations of the same moiety and may help inform dose adjustments for LCPT in kidney transplantation.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
J.D. Momper has received consulting fees from Veloxis Pharmaceuticals, Inc. B.M. Masters and S.J. Patel are employees of Veloxis Pharmaceuticals, Inc. R. Venkataramanan, A.S. Jantz, D.M. Cibrik, and K. Birdwell have no conflicts of interest to declare.
Figures
References
-
- Gidal BE, Clark AM, Anders B, et al. The application of half-life in clinical decision making: comparison of the pharmacokinetics of extended-release topiramate (USL255) and immediate-release topiramate. Epilepsy Res. 2017;129:26–32. - PubMed
-
- Dutta S, Reed RC. Functional half-life is a meaningful descriptor of steady-state pharmacokinetics of an extended-release formulation of a rapidly cleared drug: as shown by once-daily divalproex-ER. Clin Drug Investig. 2006;26:681–690. - PubMed
-
- Boxenbaum H, Battle M. Effective half-life in clinical pharmacology. J Clin Pharmacol. 1995;35:763–766. - PubMed
-
- Bialer M, Soares-da-Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia. 2012;53:935–946. - PubMed

