Prostate Cancer Risk Stratification in NRG Oncology Phase III Randomized Trials Using Multimodal Deep Learning With Digital Histopathology
- PMID: 39447096
- PMCID: PMC11520341
- DOI: 10.1200/PO.24.00145
Prostate Cancer Risk Stratification in NRG Oncology Phase III Randomized Trials Using Multimodal Deep Learning With Digital Histopathology
Abstract
Purpose: Current clinical risk stratification methods for localized prostate cancer are suboptimal, leading to over- and undertreatment. Recently, machine learning approaches using digital histopathology have shown superior prognostic ability in phase III trials. This study aims to develop a clinically usable risk grouping system using multimodal artificial intelligence (MMAI) models that outperform current National Comprehensive Cancer Network (NCCN) risk groups.
Materials and methods: The cohort comprised 9,787 patients with localized prostate cancer from eight NRG Oncology randomized phase III trials, treated with radiation therapy, androgen deprivation therapy, and/or chemotherapy. Locked MMAI models, which used digital histopathology images and clinical data, were applied to each patient. Expert consensus on cut points defined low-, intermediate-, and high-risk groups on the basis of 10-year distant metastasis rates of 3% and 10%, respectively. The MMAI's reclassification and prognostic performance were compared with the three-tier NCCN risk groups.
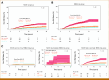
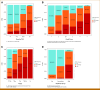
Results: The median follow-up for censored patients was 7.9 years. According to NCCN risk categories, 30.4% of patients were low-risk, 25.5% intermediate-risk, and 44.1% high-risk. The MMAI risk classification identified 43.5% of patients as low-risk, 34.6% as intermediate-risk, and 21.8% as high-risk. MMAI reclassified 1,039 (42.0%) patients initially categorized by NCCN. Despite the MMAI low-risk group being larger than the NCCN low-risk group, the 10-year metastasis risks were comparable: 1.7% (95% CI, 0.2 to 3.2) for NCCN and 3.2% (95% CI, 1.7 to 4.7) for MMAI. The overall 10-year metastasis risk for NCCN high-risk patients was 16.6%, with MMAI further stratifying this group into low-, intermediate-, and high-risk, showing metastasis rates of 3.4%, 8.2%, and 26.3%, respectively.
Conclusion: The MMAI risk grouping system expands the population of men identified as having low metastatic risk and accurately pinpoints a high-risk subset with elevated metastasis rates. This approach aims to prevent both overtreatment and undertreatment in localized prostate cancer, facilitating shared decision making.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- D'Amico AV, Whittington R, Malkowicz SB, et al. : Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969-974, 1998 - PubMed
-
- Klein EA, Haddad Z, Yousefi K, et al. : Decipher genomic classifier measured on prostate biopsy predicts metastasis risk. Urology 90:148-152, 2016 - PubMed
-
- Tward JD, Schlomm T, Bardot S, et al. : Personalizing localized prostate cancer: Validation of a combined clinical cell-cycle risk (CCR) score threshold for prognosticating benefit from multimodality therapy. Clin Genitourin Cancer 19:296-304 e3, 2021 - PubMed
-
- Dess RT, Suresh K, Zelefsky MJ, et al. : Development and validation of a clinical prognostic stage group system for nonmetastatic prostate cancer using disease-specific mortality results from the International staging Collaboration for cancer of the prostate. JAMA Oncol 6:1912-1920, 2020 - PMC - PubMed

