Sex differences in the adrenal circadian clock: a role for BMAL1 in the regulation of urinary aldosterone excretion and renal electrolyte balance in mice
- PMID: 39447118
- PMCID: PMC12352493
- DOI: 10.1152/ajprenal.00177.2024
Sex differences in the adrenal circadian clock: a role for BMAL1 in the regulation of urinary aldosterone excretion and renal electrolyte balance in mice
Abstract
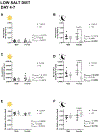
Brain and muscle ARNT-Like 1 (BMAL1) is a circadian clock transcription factor that regulates physiological functions. Male adrenal-specific Bmal1 (ASCre/+::Bmal1) KO mice displayed blunted serum corticosterone rhythms, altered blood pressure rhythm, and altered timing of eating, but there is a lack of knowledge in females. This study investigates the role of adrenal BMAL1 in renal electrolyte handling and urinary aldosterone levels in response to low salt in male and female mice. Mice were placed in metabolic cages to measure 12-h urinary aldosterone after a standard diet and 7 days low-salt diet, as well as daily body weight, 12-h food and water intake, and renal sodium and potassium balance. Adrenal glands and kidneys were collected at ZT0 or ZT12 to measure the expression of aldosterone synthesis genes and clock genes. Compared with littermate controls, ASCre/+::Bmal1 KO male and female mice displayed increased urinary aldosterone in response to a low-salt diet, although mRNA expression of aldosterone synthesis genes was decreased. Timing of food intake was altered in ASCre/+::Bmal1 KO male and female mice, with a blunted night/day ratio. ASCre/+::Bmal1 KO female mice displayed decreases in renal sodium excretion in response to low salt, but both male and female KO mice had changes in sodium balance that were time-of-day-dependent. In addition, sex differences were found in adrenal and kidney clock gene expression. Notably, this study highlights sex differences in clock gene expression that could contribute to sex differences in physiological functions.NEW & NOTEWORTHY Our findings highlight the importance of sex as well as time-of-day in understanding the role of the circadian clock in the regulation of homeostasis. Time-of-day is a key biological variable that is often ignored in research, particularly in preclinical rodent studies. Our findings demonstrate important differences in several measures at 6 AM compared with 6 PM. Consideration of time-of-day is critical for the translation of findings in nocturnal rodent physiology to diurnal human physiology.
Keywords: adrenal gland; brain and muscle ARNT-like 1; circadian rhythms; kidney; sex differences.
Copyright © 2025 The Authors.
Figures

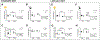





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

