Unfractionated Heparin Enhances Sepsis Prognosis Through Inhibiting Drp1-Mediated Mitochondrial Quality Imbalance
- PMID: 39447130
- PMCID: PMC11633531
- DOI: 10.1002/advs.202407705
Unfractionated Heparin Enhances Sepsis Prognosis Through Inhibiting Drp1-Mediated Mitochondrial Quality Imbalance
Abstract
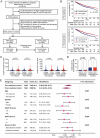
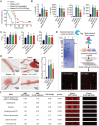
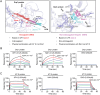
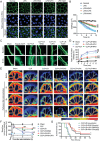
Unfractionated heparin (UFH) is commonly used as an anticoagulant in sepsis treatment and has recently been found to have non-anticoagulant effects, but underlying mechanisms remain unclear. This retrospective clinical data showed that UFH has significant protective effects in sepsis compared to low-molecular-weight heparin and enoxaparin, indicating potential benefits of its non-anticoagulant properties. Recombinant protein chip screening, surface plasmon resonance, and molecular docking data demonstrated that UFH specifically bound to the cytoplasmic Drp1 protein through its zone 2 non-anticoagulant segment. In-vitro experiments verified that UFH's specific binding to Drp1 suppressed Drp1 translocation to mitochondria following "sepsis" challenge, thereby improving mitochondrial morphology, function and metabolism in vascular endothelial cells. Consequently, UHF comprehensively protected mitochondrial quality, thus reducing vascular leakage and improving prognosis in a sepsis rat model. These findings highlight the potential of UFH as a sepsis treatment strategy targeting non-anticoagulation mechanism.
Keywords: Drp1; endothelial dysfunction; mitochondria quality; sepsis; unfractionated heparin.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cecconi M., Evans L., Levy M., Rhodes A., Lancet 2018, 392, 75. - PubMed
-
- Rudd K. E., Johnson S. C., Agesa K. M., Shackelford K. A., Tsoi D., Kievlan D. R., Colombara D. V., Ikuta K. S., Kissoon N., Finfer S., Fleischmann‐Struzek C., Machado F. R., Reinhart K. K., Rowan K., Seymour C. W., Watson R. S., West T. E., Marinho F., Hay S. I., Lozano R., Lopez A. D., Angus D. C., Murray C. J. L., Naghavi M., Lancet 2020, 395, 200. - PMC - PubMed
-
- Ranieri V. M., Thompson B. T., Barie P. S., Dhainaut J.‐F., Douglas I. S., Finfer S., Gårdlund B., Marshall J. C., Rhodes A., Artigas A., Payen D., Tenhunen J., Al‐Khalidi H. R., Thompson V., Janes J., Macias W. L., Vangerow B., Williams M. D., N. Engl. J. Med. 2012, 366, 2055. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
