Human monoclonal antibody cloning and expression with overlap extension PCR and short DNA fragments
- PMID: 39447635
- PMCID: PMC11585411
- DOI: 10.1016/j.jim.2024.113768
Human monoclonal antibody cloning and expression with overlap extension PCR and short DNA fragments
Abstract
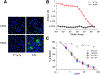
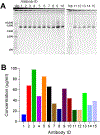
Monoclonal antibodies are powerful therapeutic, diagnostic, and research tools. Methods utilized to generate monoclonal antibodies are evolving rapidly. We created a transfectable linear antibody expression cassette from a 2-h high-fidelity overlapping PCR reaction from synthesized DNA fragments. We coupled heavy and light chains into a single linear sequence with a promoter, self-cleaving peptide, and poly(A) signal to increase the flexibility of swapping variable regions from any sequence available in silico. Transfection of the linear cassette tended to generate similar levels to the two-plasmid system and generated an average of 47 μg (14-98 μg) after 5 days in 2 ml cultures with 15 unique antibody sequences. The levels of antibodies produced were sufficient for most downstream applications in less than a week. The method presented here reduces the time, cost, and complexity of cloning steps.
Keywords: B cells; Biolayer interferometry; Confocal microscopy; Influenza; Monoclonal antibodies.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare to have no financial and non-financial competing interests.
Figures
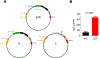



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

