Three-Year Efficacy and Safety of Mirikizumab Following 152 Weeks of Continuous Treatment for Ulcerative Colitis: Results From the LUCENT-3 Open-Label Extension Study
- PMID: 39448057
- PMCID: PMC12235137
- DOI: 10.1093/ibd/izae253
Three-Year Efficacy and Safety of Mirikizumab Following 152 Weeks of Continuous Treatment for Ulcerative Colitis: Results From the LUCENT-3 Open-Label Extension Study
Abstract
Background: Mirikizumab, a p19-directed interleukin-23 monoclonal antibody, has demonstrated induction of clinical remission at week 12 with maintenance through week 104 in patients with moderately-to-severely active ulcerative colitis (UC). Results are presented from the LUCENT-3 open-label extension study through week 152.
Methods: Of 868 LUCENT clinical trial program mirikizumab-treated induction patients, 544 were responders of whom 365 were rerandomized to mirikizumab maintenance. Of these, 324 completed week 52 and 316 entered extension treatment (286 week 52 responders; 179 week 52 remitters). Efficacy and safety outcomes are reported for mirikizumab-treated LUCENT-3 participants, including biologic-failed patients, with data for week 52 maintenance responders/remitters. Discontinuations or missing data were handled by nonresponder imputation, modified nonresponder imputation (mNRI), and observed cases.
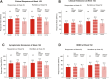
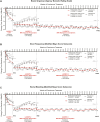
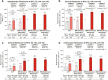
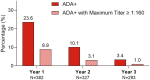
Results: Using mNRI, 81.6% of week 52 responders demonstrated clinical response at week 152. Week 152 remission rates for week 52 responders included clinical (56.1%), corticosteroid-free (CSF; 54.5%), endoscopic (61.0%), histologic-endoscopic mucosal remission (HEMR; 52.6%), symptomatic (74.9%), and bowel urgency (BU; 58.6%). At week 152, 53.3% of week 52 responders achieved histologic-endoscopic mucosal improvement (HEMI) and 74.3% achieved BU clinically meaningful improvement (CMI). Among week 52 remitters, 85.4% showed a clinical response at week 152, with clinical (70.1%), CSF (68.9%), endoscopic (72.0%), HEMR (63.4%), symptomatic (81.4%), and BU (60.8%) remission. At week 152, among week 52 remitters, 64.0% of patients achieved HEMI and 75.6% achieved BU CMI. Stool frequency, rectal bleeding, BU, and abdominal pain score reductions from induction baseline to maintenance week 52 were sustained through week 152 for week 52 completers. Overall, in the safety population, 7.4% of patients reported severe adverse events (AEs); 5.3% discontinued treatment due to AEs. AEs of special interest included opportunistic infection (1.8%), hepatic disorders (3.2%), cerebrocardiovascular events (1.5%), and malignancy (0.3%). Patients with antidrug antibodies reduced over time from 23.6% in year 1 to 3.2% in year 3.
Conclusions: Symptomatic, clinical, endoscopic, histologic, and quality-of-life outcomes support long-term sustained benefit of mirikizumab treatment up to 152 weeks in patients with UC, including biologic-failed patients, with no new safety concerns.
Clinical trial registry: ClinicalTrials.gov: NCT03518086; NCT03524092; NCT03519945.
Keywords: interleukin-23 p19 antibody; long-term extension; mirikizumab; ulcerative colitis; week 152 results.
Plain language summary
Long-term symptomatic, clinical response/remission, endoscopic, and histologic data from an open-label study of patients with moderately-to-severely active ulcerative colitis demonstrate that 3-year continuous treatment with mirikizumab maintained clinical remission in most induction clinical responders, regardless of previous biologic failure status.
© 2024 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
B.E.S. reports consulting fees from AbbVie, Alimentiv, Amgen, Arena Pharmaceuticals, Artugen Therapeutics, AstraZeneca, Biora Therapeutics, Inc., Boehringer Ingelheim, Boston Pharmaceuticals, Calibr, Celgene, Celltrion, ClostraBio, Enthera, Equillium, Evommune, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Innovation Pharmaceuticals Inc, Inotrem, Kaleido Biosciences, Kallyope, Merck & Co., Inc., Morphic Therapeutic, MRM Health, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics Inc, Q32 Bio, Sun Pharma, Surrozen, Target RWE, Teva Pharmaceuticals, TLL Pharmaceutical LLC, and Ventyx Biosciences; consulting and speaking fees from Abivax; consulting and speaking fees and other support from Eli Lilly and Company; research grants, consulting and speaking fees, and other support from Bristol Myers Squibb, Janssen, Pfizer, and Takeda Pharmaceuticals; research grants and consulting fees from Theravance Biopharma; and stock options from Ventyx Biosciences. G.D. reports advisor fees from AbbVie, Alimentiv, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Eli Lilly and Company, Galapagos, GlaxoSmithKline, Gossamer Bio, Immunic Therapeutics, Johnson & Johnson, Pfizer, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics Inc, Samsung Biologics, Seres Therapeutics, Takeda Pharmaceuticals, Tillotts Pharma AG, and Ventyx Biosciences. D.B.C., J.T.J., T.H.G., R.E.M., and J.M. are employees and stockholders of Eli Lilly and Company. P.M.I. reports research grants from Celltrion, Galapagos, Pfizer, and Takeda Pharmaceuticals; consulting fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Elasmogen, Eli Lilly and Company, Gilead Sciences, Janssen, Pfizer, Prometheus, and Sandoz; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Bristol Myers Squibb, Celgene, Celltrion, Dr. Falk Pharma, Eli Lilly and Company, Galapagos, Gilead Sciences, Janssen, Pfizer, Takeda Pharmaceuticals, and Tillotts Pharma AG; and support for attending meetings and/or travel from AbbVie and Tillotts Pharma AG. M.T.A. reports consulting and/or serving on an advisory board for AbbVie, Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Celsius Therapeutics, Eli Lilly and Company, Gilead Sciences, Janssen, Pfizer, Prometheus Biosciences, and UCB; and teaching, lecturing, or speaking from Alimentiv. S.L. reports grants and research support from AbbVie, Arena Pharmaceuticals, Atlantic Pharmaceuticals, Celgene, Gilead Sciences, Janssen, Pfizer, Salix Pharmaceuticals, Shield Therapeutics, Takeda Pharmaceuticals, Tetherex Pharmaceuticals, and UCB; and consulting for Arena Pharmaceuticals, Celgene, Celltrion, Cornerstone Pharmaceuticals, Eli Lilly and Company, Janssen, Mesoblast, Pfizer, Salix Pharmaceuticals, Takeda Pharmaceuticals, and UCB. T.H. reports lecture fees from AbbVie, EA Pharma, Gilead Sciences, Janssen, JIMRO, Kyorin Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., Pfizer, and Takeda Pharmaceuticals; advisory/consultancy fees from AbbVie, EA Pharma, Eli Lilly and Company, Gilead Sciences, Janssen, Mitsubishi Tanabe Pharma Corporation, Pfizer, and Takeda Pharmaceuticals; and pharmaceutical/research grants from AbbVie, Alfresa Pharma, Daiichi Sankyo, EA Pharma, JIMRO, Kyorin Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., Nichi-lko Pharmaceutical Co., Ltd., Nippon Kayaku, Pfizer, Takeda Pharmaceuticals, and Zeria Pharmaceutical Co., Ltd. T.K. reports serving as a speaker, consultant, or advisory board member for AbbVie, Alfresa Pharma, Bristol Myers Squibb, Celltrion, Covidien, EA Pharma, Eiken Chemical Co., Ltd., Eli Lilly and Company, Ferring Pharmaceuticals, Gilead Sciences, Janssen, JIMRO, Kissei Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., Nippon Kayaku, Pfizer, Sekisui Medical Co., Ltd., Takeda Pharmaceuticals, and Zeria Pharmaceutical Co., Ltd.; research funding from AbbVie, Alfresa Pharma, EA Pharma, Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku, Otsuka Holdings Co., Ltd., Pfizer, Sekisui Medical Co., Ltd., and Zeria Pharmaceutical Co., Ltd. M.C.D. reports consulting fees from AbbVie, Arena Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Genentech (Roche), Gilead Sciences, Janssen, Pfizer, Prometheus Biosciences, Roche, Takeda Pharmaceuticals, and UCB; contracted research from AbbVie and Janssen; stock interest in Trellus Health; and licensing fees from Takeda Pharmaceuticals. S.V. reports grants from AbbVie, Galapagos, Johnson & Johnson, Pfizer, and Takeda Pharmaceuticals; and consulting and/or speaking fees from AbbVie, Abivax, AbolerIS Pharma, Agomab Therapeutics, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia Biologics, Biora Therapeutics, Inc., Bristol Myers Squibb, Boehringer Ingelheim, Celgene, CVasThera, Cytoki Pharma, Dr. Falk Pharma, Eli Lilly and Company, Ferring Pharmaceuticals, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Hospira, IMIDomics, Janssen, Johnson & Johnson, Materias Primas Farmacéuticas, Mestag Therapeutics, MiroBio, Morphic Therapeutic, MRM Health, Mundipharma, MSD, Pfizer, ProDigest, Prometheus, Second Genome, Shire Pharma, Surrozen, Takeda Pharmaceuticals, Theravance Biopharma, Tillotts Pharma AG, VectivBio, Ventyx Biosciences, and Zealand Pharma. C.A.S. reports consulting for AbbVie, Bristol Myers Squibb, Eli Lilly and Company, Fresenius Kabi, Janssen, Napo Pharmaceuticals, Pfizer, ProciseDx, Prometheus, Takeda Pharmaceuticals, and Trellus Health; speaker for continuing medical education activities for AbbVie, Janssen, Pfizer, and Takeda Pharmaceuticals; and grant support from AbbVie, Janssen, Pfizer, and Takeda Pharmaceuticals. L.P.-B. reports personal fees from AbbVie, Abivax, Adacyte Therapeutics, Alimentiv, Alma Bio Therapeutics, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Biogen, Bristol Myers Squibb, Celltrion, Connect Biopharma, Cytoki Pharma, Eli Lilly and Company, Enthera, Ferring Pharmaceuticals, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, H.A.C. Pharma, IAG Image Analysis Group, Index Pharmaceuticals, Inotrem, Janssen, Medac Pharma, Mopac Biologics, Morphic Therapeutic, MSD, Nordic Pharma, Norgine, Novartis, OM Pharma, ONO Pharmaceutical Co., Ltd., OSE Immunotherapeutics, Pandion Therapeutics, Par’Immune, Pfizer, Prometheus Biosciences, Protagonist Therapeutics Inc, Roche, Roivant, Samsung Biologics, Sandoz, Sanofi, Takeda Pharmaceuticals, Theravance Biopharma, Thermo Fisher Scientific, TiGenix, Tillotts Pharma AG, VectivBio, Ventyx Bioscience, Viatris, Vifor Pharma, and YSOPIA Bioscience. R.P. reports acting as a consultant for Abbott, AbbVie, Abivax, Alimentiv, Amgen, Arena Pharmaceuticals, AstraZeneca, Biogen, Biora Therapeutics, Inc., Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Cosmo Pharmaceuticals, Eisai, Elan Pharmaceuticals, Eli Lilly and Company, Ferring Pharmaceuticals, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, JAMP Pharma, Janssen, Merck & Co., Inc., Novartis, Oppilan Pharma, Organon, Pandion Therapeutics, Pendopharm G.I. Solutions, Pfizer, Prometheus Biosciences, Protagonist Therapeutics Inc, Roche, Sandoz, Satisfai Health, Shire Pharma, Sublimity Therapeutics, Takeda Pharmaceuticals, Theravance Biopharma, Trellus Health, Ventyx Biosciences, Viatris, and UCB; speaker’s fees from AbbVie, Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Ferring Pharmaceuticals, Fresenius Kabi, Gilead Sciences, Janssen, Merck & Co., Inc., Organon, Pfizer, Roche, Sandoz, Shire Pharma, and Takeda Pharmaceuticals; and advisory boards for AbbVie, Alimentiv, Amgen, Arena Pharmaceuticals, AstraZeneca, Biogen, Biora Therapeutics, Inc., Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Ferring Pharmaceuticals, Fresenius Kabi, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, JAMP Pharma, Janssen, Merck & Co., Inc., Novartis, Organon, Pandion Therapeutics, Pfizer, Protagonist Therapeutics Inc, Roche, Sandoz, Shire Pharma, Sublimity Therapeutics, Takeda Pharmaceuticals, Ventyx Biosciences, and Viatris. A.D. reports received fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis, and end point committees from AbbVie, Abivax, Arena Pharmaceuticals, Bristol Myers Squibb, Celgene, Falk Foundation, Galapagos, Gilead Sciences, Janssen, and Pfizer; consultancy fees from AbbVie, Amgen, Arena Pharmaceuticals, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Eli Lilly and Company, Falk Foundation, Ferring Pharmaceuticals, Fresenius Kabi, Galapagos, Genentech (Roche), Janssen, MSD, Pfizer, Pharmacosmos, Sandoz/Hexal, Takeda Pharmaceuticals, Tillotts Pharma AG, and Vifor Pharma; payment from lectures including service on speakers bureaus from AbbVie, Biogen, CED Service GmbH, Celltrion, Falk Foundation, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, High5MD, Janssen, Materias Primas Farmacéuticas, MedToday, MSD, Pfizer, Takeda Pharmaceuticals, Tillotts Pharma AG, and Vifor Pharma; and payment for manuscript preparation from Falk Foundation, Takeda Pharmaceuticals, Thieme, and UNI-MED Verlag AG.
Figures


References
-
- Gros B, Kaplan GG.. Ulcerative colitis in adults: a review. JAMA. 2023;330(10):951-965. doi: https://doi.org/ 10.1001/jama.2023.15389 - DOI - PubMed
-
- Dubinsky MC, Watanabe K, Molander P, et al. Ulcerative colitis narrative global survey findings: the impact of living with ulcerative colitis-patients’ and physicians’ view. Inflamm Bowel Dis. 2021;27(11):1747-1755. doi: https://doi.org/ 10.1093/ibd/izab016 - DOI - PMC - PubMed
-
- Le Berre C, Honap S, Peyrin-Biroulet L.. Ulcerative colitis. Lancet. 2023;402(10401):571-584. doi: https://doi.org/ 10.1016/S0140-6736(23)00966-2 - DOI - PubMed
-
- Steere B, Schmitz J, Powell N, et al. Mirikizumab regulates genes involved in ulcerative colitis disease activity and anti-TNF resistance: results from a phase 2 study. Clin Transl Gastroenterol. 2023;14(7):e00578. doi: https://doi.org/ 10.14309/ctg.0000000000000578 - DOI - PMC - PubMed
-
- Danese S, Allez M, van Bodegraven AA, et al. Unmet medical needs in ulcerative colitis: an expert group consensus. Dig Dis. 2019;37(4):266-283. doi: https://doi.org/ 10.1159/000496739 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

