Kinetic Resolution of BINOLs and Biphenols by Atroposelective, Cu-H-Catalyzed Si-O Coupling with Hydrosilanes
- PMID: 39448059
- PMCID: PMC11555675
- DOI: 10.1021/acs.orglett.4c03557
Kinetic Resolution of BINOLs and Biphenols by Atroposelective, Cu-H-Catalyzed Si-O Coupling with Hydrosilanes
Abstract
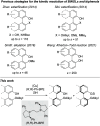
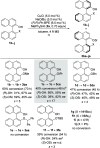
A nonenzymatic kinetic resolution of monoprotected BINOL and biphenol derivatives by atroposelective Si-O coupling with hydrosilanes is described. The reaction relies on a previously unprecedented Cu-H-catalyzed silylation of phenols. The catalyst system consisting of CuCl, (R,R)-Ph-BPE, and NaOtBu enables the enantioselective coupling of the phenolic hydroxy group with a hydrosilane with moderate to good selectivity factors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Hoveyda A. H.; Snapper M. L.. Enantioselective Synthesis of Silyl Ethers Through Catalytic Si–O Bond Formation. In Organosilicon Chemistry: Novel Approaches and Reactions; Hiyama T., Oestreich M., Eds.; Wiley-VCH: 2019; pp 459–493.
- Seliger J.; Oestreich M. Making the Silylation of Alcohols Chiral: Asymmetric Protection of Hydroxy Groups. Chem. - Eur. J. 2019, 25, 9358–9365. 10.1002/chem.201900792. - DOI - PubMed
- Xu L.-W.; Chen Y.; Lu Y. Catalytic Silylations of Alcohols: Turning Simple Protecting-Group Strategies into Powerful Enantioselective Synthetic Methods. Angew. Chem., Int. Ed. 2015, 54, 9456–9466. 10.1002/anie.201504127. - DOI - PubMed
-
- Isobe T.; Fukuda K.; Araki Y.; Ishikawa T. Modified guanidines as chiral superbases: the first example of asymmetric silylation of secondary alcohols. Chem. Commun. 2001, 243–244. 10.1039/b009173l. - DOI
- Zhao Y.; Mitra A. W.; Hoveyda A. H.; Snapper M. L. Kinetic Resolution of 1,2-Diols through Highly Site- and Enantioselective Catalytic Silylation. Angew. Chem., Int. Ed. 2007, 46, 8471–8474. 10.1002/anie.200703650. - DOI - PubMed
- Worthy A. D.; Sun X.; Tan K. L. Site-Selective Catalysis: Toward a Regiodivergent Resolution of 1,2-Diols. J. Am. Chem. Soc. 2012, 134, 7321–7234. 10.1021/ja3027086. - DOI - PMC - PubMed
- Sun X.; Worthy A. D.; Tan K. L. Resolution of Terminal 1,2-Diols via Silyl Transfer. J. Org. Chem. 2013, 78, 10494–10499. 10.1021/jo4012909. - DOI - PMC - PubMed
- Sheppard C. I.; Taylor J. L.; Wiskur S. L. Silylation-Based Kinetic Resolution of Monofunctional Secondary Alcohols. Org. Lett. 2011, 13, 3794–3797. 10.1021/ol2012617. - DOI - PubMed
- Clark R. W.; Deaton T. M.; Zhang Y.; Moore M. I.; Wiskur S. L. Silylation-Based Kinetic Resolution of α-Hydroxy Lactones and Lactams. Org. Lett. 2013, 15, 6132–6135. 10.1021/ol402982w. - DOI - PubMed
- Yoshimatsu S.; Nakata K. Silylative Kinetic Resolution of Racemic 2,2-Dialkyl 5- and 6-Membered Cyclic Benzylic Alcohol Derivatives Catalyzed by Chiral Guanidine, (R)-N-Methylbenzoguanidine. Adv. Synth. Catal. 2019, 361, 4679–4684. 10.1002/adsc.201900761. - DOI
-
- Rendler S.; Auer G.; Oestreich M. Kinetic Resolution of Chiral Secondary Alcohols by Dehydrogenative Coupling with Recyclable Silicon-Stereogenic Silanes. Angew. Chem., Int. Ed. 2005, 44, 7620–7624. 10.1002/anie.200502631. - DOI - PubMed
- Weickgenannt A.; Mewald M.; Muesmann T. W. T.; Oestreich M. Catalytic Asymmetric Si–O Coupling of Simple Achiral Silanes and Chiral Donor-Functionalized Alcohols. Angew. Chem., Int. Ed. 2010, 49, 2223–2226. 10.1002/anie.200905561. - DOI - PubMed
LinkOut - more resources
Full Text Sources

