Impact of select actionable genomic alterations on efficacy of neoadjuvant immunotherapy in resectable non-small cell lung cancer
- PMID: 39448200
- PMCID: PMC11499765
- DOI: 10.1136/jitc-2024-009677
Impact of select actionable genomic alterations on efficacy of neoadjuvant immunotherapy in resectable non-small cell lung cancer
Abstract
Background: Neoadjuvant immune checkpoint inhibitors (ICIs) have improved survival outcomes compared with chemotherapy in resectable non-small cell lung cancer (NSCLC). However, the impact of actionable genomic alterations (AGAs) on the efficacy of neoadjuvant ICIs remains unclear. We report the influence of AGAs on treatment failure (TF) in patients with resectable NSCLC treated with neoadjuvant ICIs.
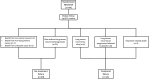
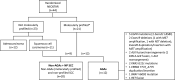
Methods: Tumor molecular profiles were obtained from patients with stage I-IIIA resectable NSCLC (American Joint Committee on Cancer seventh edition) treated with either neoadjuvant nivolumab (N, n=23) or nivolumab+ipilimumab (NI, n=21) followed by surgery in a previously reported phase-2 randomized study (NCT03158129). TF was defined as any progression of primary lung cancer after neoadjuvant ICI therapy in patients without surgery, radiographic and/or biopsy-proven primary lung cancer recurrence after surgery, or death from possibly treatment-related complications or from primary lung cancer since randomization. Tumors with AGAs (n=12) were compared with tumors without AGAs and non-profiled squamous cell carcinomas (non-AGAs+NP SCC, n=20).
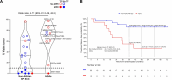
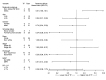
Results: With a median follow-up of 60.2 months, the overall TF rate was 34.1% (15/44). Tumor molecular profiling was retrospectively obtained in 47.7% (21/44) of patients and select AGAs were identified in 12 patients: 5 epidermal growth factor receptor (EGFR), 2 KRAS, 1 ERBB2, and 1 BRAF mutations, 2 anaplastic lymphoma kinase (ALK) and 1 RET fusions. The median time to TF in patients with AGAs was 24.7 months (95% CI: 12.6 to 40.4), compared with not reached (95% CI: not evaluable (NE)-NE) in the non-AGAs+NP SCC group. The TF risk was higher in AGAs (HR: 5.51, 95% CI: 1.68 to 18.1), and lower in former/current smokers (HR: 0.24, 95% CI: 0.08 to 0.75). The odds of major pathological response were 4.71 (95% CI: 0.49 to 45.2) times higher in the non-AGAs+NP SCC group, and the median percentage of residual viable tumor was 72.5% in AGAs compared with 33.0% in non-AGS+NP SCC tumors.
Conclusions: Patients with NSCLC harboring select AGAs, including EGFR and ALK alterations, have a higher risk for TF, shorter median time to TF, and diminished pathological regression after neoadjuvant ICIs. The suboptimal efficacy of neoadjuvant chemotherapy-sparing, ICI-based regimens in this patient subset underscores the importance of tumor molecular testing prior to initiation of neoadjuvant ICI therapy in patients with resectable NSCLC.
Keywords: Immunotherapy; Ipilimumab; Lung Cancer; Nivolumab; Recurrence.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NZ reports consulting fee, travel, dining expenses from Ethicon. WW reports consulting fees, speaker fees and/or honoraria from Amgen, AstraZeneca, Genentech/Roche, Astellas, Boehringer Ingelheim, Merck, Eli Lilly, BMS, MSD, Bayer, Pfizer, Janssen, Sanofi-Aventis, Takeda, Novartis, United Medical; and research grants (to institution) from Amgen, AstraZeneca, Genentech/Roche, Astellas, Boehringer Ingelheim, Eli Lilly, BMS, MSD, Pfizer, Janssen, Sanofi-Aventis. GRB reports grants or contracts from Amgen, Bayer, Adaptimmune, Exelixis, Daiichi Sankyo, GlaxoSmithKline, Immatics, Immunocore, Incyte, Kite Pharma, Macrogenics, Torque, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, MedImmune, Merck, Novartis, Roche, Sanofi, Xcovery, Tmunity Therapeutics, Regeneron, BeiGene, Repertoire Immune Medicines, Verastem. CytomX Therapeutics, Duality Biologics, Mythic Therapeutics, Takeda, Aulos Bioscience, Seagen, Nuvalent, Turning Point Therapeutics; Consulting fees from AbbVie, Adicet, Amgen, Ariad, Bayer, Clovis Oncology, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Instil Bio, Genentech, Genzyme, Gilead, Lilly, Janssen, MedImmune, Merck, Novartis, Roche, Sanofi, Tyme Oncology, Xcovery, Virogin Biotech, Maverick Therapeutics, BeiGene, Regeneron, CytomX Therapeutics, Intervenn Biosciences, Onconova Therapeutics, Seagen, Scorpion Therapeutics, Immunocore. Advisory board for Virogin Biotech SAB, Beigene, Immunocore, Regeneron. XL reports consulting/advisory fees from Eli Lilly, EMD Serono (Merck KGaA), AstraZeneca, Spectrum Pharmaceutics, Novartis, Regeneron, Boehringer Ingelheim, Hengrui Therapeutics, Bayer, Teligene, Taiho, Daiichi Sankyo, Janssen, Blueprint Medicines, Sensei Biotherapeutics, SystImmune, ArriVent, Abion, and AbbVie; Research Funding to Institution from Eli Lilly, EMD Serono, ArriVent, Dizal, Teligene, Regeneron, Janssen, ThermoFisher, Takeda, and Boehringer Ingelheim; Travel support from EMD Serono, Janssen, and Spectrum Pharmaceutics. MA reports research funding from Genentech, Nektar Therapeutics, Merck, GlaxoSmithKline, Novartis, Jounce Therapeutics, Bristol Myers Squibb, Eli Lilly, Adaptimmune, Shattuck Labs, Gilead; Consulting fees from GlaxoSmithKline, Shattuck Labs, Bristol Myers Squibb, AstraZeneca, Insight; Speaker fees from AstraZeneca, Nektar Therapeutics, SITC; and participation of safety review committee for Nanobiotix-MDA Alliance, Henlius. JAR reports consulting fee, stock, patents issued and pending with Genprex. MVN reports receiving research funding to institution from Mirati, Novartis, Checkmate, Alaunos, AstraZeneca, Pfizer, Genentech, Navire; a consultant or advisory role for Mirati, Merck/MSD, Novartis, Genentech, Sanofi, Pfizer; and other support from Ziopharm Oncology, ApotheCom, Ashfield Healthcare. HTT reports consulting fee for Abion Bio. JZ reports grants from Merck, Helius, grants and personal fees from Johnson and Johnson and Novartis, personal fees from Bristol Myers Squibb, AstraZeneca, GenePlus, Innovent, Varian, Catalyst and Hengrui outside the submitted work. DLG has served on scientific advisory committees for Menarini Ricerche, 4D Pharma, Onconova, and Eli Lilly and has received research support from Takeda, Astellas, NGM Biopharmaceuticals, Boehringer Ingelheim and Mirati. JVH reports Advisory Committees—AbbVie, AnHeart Therapeutics, AstraZeneca, BioNTech AG, BI, BMS, DAVA Oncology, Eli Lily Research Support—AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb and Takeda; Licensing/Royalties—Spectrum. BS reports consulting fees from AstraZeneca, BMS, Medscape; dining from AstraZeneca. BMS; Speaker fees from AstraZeneca, Medscape, Peer View; advisory board from AstraZeneca, BMS. TC reports (over the past 24 months) speaker fees/honoraria (including travel/meeting expenses) from ASCO Post, AstraZeneca, Bio Ascend, Bristol Myers Squibb, Clinical Care Options, IDEOlogy Health, Medical Educator Consortium, Medscape, OncLive, PEAK Medicals, PeerView, Physicians' Education Resource, Targeted Oncology; advisory role/consulting fees (including travel/meeting expenses) from AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Genentech, Merck, oNKo-innate, Pfizer, RAPT Therapeutics, Regeneron; institutional research funding from AstraZeneca and Bristol Myers Squibb.
Figures

References
-
- Awad MM, Forde PM, Girard N, et al. 1261O Neoadjuvant nivolumab (N) + ipilimumab (I) vs chemotherapy (C) in the phase III CheckMate 816 trial. Ann Oncol. 2023;34 doi: 10.1016/j.annonc.2023.09.739. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
