Alum-anchored IL-12 combined with cytotoxic chemotherapy and immune checkpoint blockade enhanced antitumor immune responses in head and neck cancer models
- PMID: 39448201
- PMCID: PMC11499830
- DOI: 10.1136/jitc-2024-009712
Alum-anchored IL-12 combined with cytotoxic chemotherapy and immune checkpoint blockade enhanced antitumor immune responses in head and neck cancer models
Abstract
Background: First-line treatment with pembrolizumab plus chemotherapy in recurrent and metastatic head and neck squamous cell carcinomas (HNSCC) has improved survival. However, the overall response rate with this standard of care regimen (SOC) remains limited. Interleukin (IL)-12 is a potent cytokine that facilitates the crosstalk between innate and adaptive immunity, making it crucial in the antitumor response. Alum-anchored murine IL-12 (mANK-101) has been demonstrated to elicit robust antitumor responses in diverse syngeneic models, which were correlated with increased immune effector functions and prolonged local retention of IL-12. This study investigates the therapeutic benefit of combining mANK-101 with SOC in the MOC1 and MOC2 murine HNSCC tumor models.
Methods: MOC1 and MOC2 tumor-bearing C57BL/6 mice were administered with a single intratumoral injection of mANK-101 and weekly intraperitoneal injections of cisplatin and α-programmed death 1 (PD-1) for 3 weeks. For MOC1, flow cytometry and cytokine array were performed to assess the immune effector functions associated with the combinational treatment. Multiplex immunofluorescence was employed to characterize the influence of the treatment on the immune architecture in the tumors. RNA analysis was implemented for in-depth examination of the macrophage and effector populations.
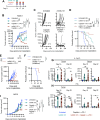
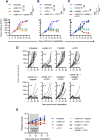
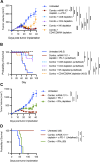
Results: In the MOC1 and MOC2 models, combination therapy with mANK-101, cisplatin, and α-PD-1 resulted in superior tumor growth inhibition and resulted in the highest rate of tumor-free survival when compared with treatment cohorts that received mANK-101 monotherapy or SOC treatment with α-PD-1 plus cisplatin. Furthermore, the combination therapy protected against tumor re-growth on rechallenge and controlled the growth of distal tumors. The improved therapeutic effect was associated with increased CD8+ T-cell recruitment, increased CD8+ and CD4+ activity, and repolarization of the macrophage population from M2 to M1 at the tumor site. Elevated and prolonged interferon-γ expression is central to the antitumor activity mediated by the combination therapy. In addition, the combination therapy with mANK-101+cisplatin+α-PD-1 induced the formation of tertiary lymphoid structure-like immune aggregates in the peritumoral space.
Conclusion: The current findings provide a rationale for the combination of alum-tethered IL-12 with cisplatin and α-PD-1 for HNSCC.
Keywords: Abscopal; Chemotherapy; Cytokine; Head and Neck Cancer; Solid Tumor.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HK and SB work for Ankyra Therapeutics.
Figures




References
-
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
