Targeting CD93 on monocytes revitalizes antitumor immunity by enhancing the function and infiltration of CD8+ T cells
- PMID: 39448202
- PMCID: PMC11499807
- DOI: 10.1136/jitc-2024-010148
Targeting CD93 on monocytes revitalizes antitumor immunity by enhancing the function and infiltration of CD8+ T cells
Erratum in
-
Correction: Targeting CD93 on monocytes revitalizes antitumor immunity by enhancing the function and infiltration of CD8+ T cells.J Immunother Cancer. 2024 Nov 11;12(11):e010148corr1. doi: 10.1136/jitc-2024-010148corr1. J Immunother Cancer. 2024. PMID: 39532434 Free PMC article. No abstract available.
Abstract
Background: Limited activation and infiltration of CD8+ T cells are major challenges facing T cell-based immunotherapy for most solid tumors, of which the mechanism is multilayered and not yet fully understood.
Methods: Levels of CD93 expression on monocytes from paired non-tumor, peritumor and tumor tissues of human hepatocellular carcinoma (HCC) were evaluated. The underlying mechanisms mediating effects of CD93+ monocytes on the inhibition and tumor exclusion of CD8+ T cells were studied through both in vitro and in vivo experiments.
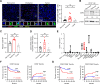
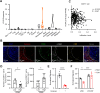
Results: In this study, we found that monocytes in the peritumoral tissues of HCC significantly increased levels of CD93 expression, and these CD93+ monocytes collocated with CD8+ T cells, whose density was much higher in peritumor than intratumor areas. In vitro experiments showed that glycolytic switch mediated tumor-induced CD93 upregulation in monocytes via the Erk signaling pathway. CD93 on the one hand could enhance PD-L1 expression through the AKT-GSK3β axis, while on the other hand inducing monocytes to produce versican, a type of matrix component which interacted with hyaluronan and collagens to inhibit CD8+ T cell migration. Consistently, levels of CD93+ monocytes positively correlated with the density of peritumoral CD8+ T cells while negatively correlated with that of intratumoral CD8+ T cells. Targeting CD93 on monocytes not only increased the infiltration and activation of CD8+ T cells but also enhanced tumor sensitivity to anti-PD-1 treatment in mice in vivo.
Conclusion: This study identified an important mechanism contributing to the activation and limited infiltration of CD8+ T cells in solid tumors, and CD93+ monocytes might represent a plausible immunotherapeutic target for the treatment of HCC.
Keywords: Hepatocellular Carcinoma; Immunotherapy; Macrophage; Monocyte; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
