MYH7-related myopathies: clinical, myopathological and genotypic spectrum in a multicentre French cohort
- PMID: 39448255
- PMCID: PMC12015026
- DOI: 10.1136/jnnp-2024-334263
MYH7-related myopathies: clinical, myopathological and genotypic spectrum in a multicentre French cohort
Abstract
Background: Myosin heavy chain 7 (MYH7)-related myopathies (MYH7-RMs) are a group of muscle disorders linked to pathogenic variants in the MYH7 gene, encoding the slow/beta-cardiac myosin heavy chain, which is highly expressed in skeletal muscle and heart. The phenotype is heterogeneous including distal, predominantly axial or scapuloperoneal myopathies with variable cardiac involvement.
Methods: We retrospectively analysed the clinical, muscle MRI, genetic and myopathological features of 57 MYH7 patients. Patients received a thorough neurological (n=57, 100%), cardiac (n=51, 89%) and respiratory (n=45, 79%) assessment. Muscle imaging findings and muscle biopsies were reappraised in 19 (33%) and 27 (47%) patients, respectively.
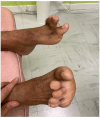
Results: We identified three phenotypes with varying degrees of overlap: distal myopathy (70%), scapuloperoneal (23%) and axial with peculiar cervical spine rigidity called the 'sphinx' phenotype (7%). 14% of patients had either dilated cardiomyopathy, hypertrophic cardiomyopathy or left ventricular non-compaction cardiomyopathy. 31% of patients had prominent respiratory involvement, including all patients with the 'sphinx' phenotype. Muscle MRI showed involvement of tibialis anterior, followed by quadriceps, and erector spinae in patients with axial phenotype. Cores represented the most common myopathological lesion. We report 26 pathogenic variants of MYH7 gene, 9 of which are novel.
Conclusions: MYH7-RMs have a large phenotypic spectrum, including distal, scapuloperoneal or axial weakness, and variable cardiac and respiratory involvement. Tibialis anterior is constantly and precociously affected both clinically and on muscle imaging. Cores represent the most common myopathological lesion. Our detailed description of MYH7-RMs should improve their recognition and management.
Keywords: GENETICS; MRI; MYOPATHY; NEUROPATHOLOGY.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
