SNX5 promotes antigen presentation in B cells by dual regulation of actin and lysosomal dynamics
- PMID: 39448266
- PMCID: PMC11502673
- DOI: 10.26508/lsa.202402917
SNX5 promotes antigen presentation in B cells by dual regulation of actin and lysosomal dynamics
Abstract
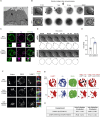
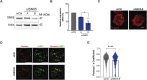
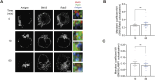
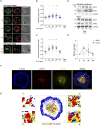
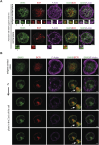
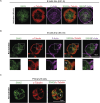
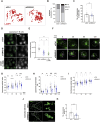
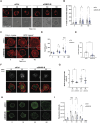
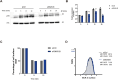
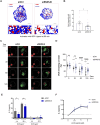
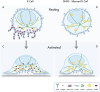
B cells rapidly adapt their endocytic pathway to promote the uptake and processing of extracellular antigens recognized through the B-cell receptor (BCR). The mechanisms coupling changes in endomembrane trafficking to the capacity of B cells to screen for antigens within lymphoid tissues remain unaddressed. We investigated the role of SNX5, a member of the sorting nexin family, which interacts with endocytic membranes to regulate vesicular trafficking and macropinocytosis. Our results show that in steady state, B cells form SNX5-rich protrusions at the plasma membrane, which dissipate upon interaction with soluble antigens, whereas B cells activated with immobilized antigens accumulate SNX5 at the immune synapse where it regulates actin-dependent spreading responses. B cells silenced for SNX5 exhibit enlarged lysosomes, which are not recruited to the synaptic membrane, decreasing their capacity to extract immobilized antigens. Overall, our findings reveal that SNX5 is critical for actin-dependent plasma membrane remodeling in B cells involved in antigen screening and immune synapse formation, as well as endolysosomal trafficking required to promote antigen extraction and presentation.
© 2024 Cabrera-Reyes et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
