Where Do Plasma Biomarkers fit in With Current Alzheimer's Disease Detection?
- PMID: 39448295
- PMCID: PMC11875946
- DOI: 10.1016/j.jagp.2024.09.015
Where Do Plasma Biomarkers fit in With Current Alzheimer's Disease Detection?
Erratum in
-
Corrigendum to "Where do plasma biomarkers fit in with current Alzheimer's disease detection?" [volume 33, issue 4, April 2025, pages 428-437].Am J Geriatr Psychiatry. 2025 Nov;33(11):1249. doi: 10.1016/j.jagp.2025.06.020. Epub 2025 Jun 27. Am J Geriatr Psychiatry. 2025. PMID: 40683789 No abstract available.
Abstract
Objectives: We examine the clinical utility of plasma-based detection for Alzheimer's disease (AD) pathophysiology in older adults with mild cognitive impairment (MCI) and whether cognitive screening can inform when to use plasma-based AD tests.
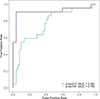
Methods: Seventy-four community-dwelling older adults with MCI had testing with plasma phosphorylated tau (p-tau) 217 and 181, positron emission tomography (PET) imaging for amyloid beta (Aβ), and cognitive assessment. Receiver operating characteristic (ROC) analysis was used to assess the diagnostic value of plasma p-tau.
Results: Plasma p-tau217 distinguished MCI participants who had PET imaging evidence of Aβ accumulation from those without (AUC of 0.92, specificity of 0.96, and sensitivity of 0.90), outperforming plasma p-tau181 (AUC of 0.76, specificity of 0.87 and sensitivity of 0.59) for the same purpose. Of the 60 MCI participants that were amnestic, 22 were Aβ+. The 14 participants that were nonamnestic were all Aβ-.
Conclusions: Our findings support the clinical use of plasma p-tau, particularly p-tau217, for patient detection of AD pathophysiology in older adults with amnestic MCI, but not in those who are nonamnestic.
Keywords: Alzheimer's disease; diagnostic tests; mild cognitive impairment; p-tau181; p-tau217.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE In the past 5 years, Dr. Lopez was a consultant for Novo Nordisk, Lundbeck, Eisai, Grifols, and Biogen. All other authors report no conflicts with any product mentioned or concept discussed in this article.
Figures

References
-
- Karikari TK, Ashton NJ, Brinkmalm G, et al. : Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat Rev Neurol 2022; 18:400–418 - PubMed
-
- Kraemer HC, Schultz SK,Arndt S: Biomarkers in psychiatry: methodological issues. Am J Geriatr Psychiatry 2002; 10:653–659 - PubMed
-
- Karikari TK, Pascoal TA, Ashton NJ, et al. : Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020; 19:422–433 - PubMed
MeSH terms
Substances
Grants and funding
- R01 AG075336/AG/NIA NIH HHS/United States
- K23 MH125074/MH/NIMH NIH HHS/United States
- R01 AG083874/AG/NIA NIH HHS/United States
- R01 AG072641/AG/NIA NIH HHS/United States
- RF1 AG025516/AG/NIA NIH HHS/United States
- R01 AG073267/AG/NIA NIH HHS/United States
- R01 AG055389/AG/NIA NIH HHS/United States
- P01 AG025204/AG/NIA NIH HHS/United States
- R37 AG023651/AG/NIA NIH HHS/United States
- U24 AG082930/AG/NIA NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- K23 AG076663/AG/NIA NIH HHS/United States
- RF1 AG052525/AG/NIA NIH HHS/United States
- R01 AG053952/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

