Mechanism of Action and Pharmacokinetics of Approved Bispecific Antibodies
- PMID: 39448393
- PMCID: PMC11535297
- DOI: 10.4062/biomolther.2024.146
Mechanism of Action and Pharmacokinetics of Approved Bispecific Antibodies
Erratum in
-
Erratum to "Mechanism of Action and Pharmacokinetics of Approved Bispecific Antibodies" [Biomol Ther 32(6), 708-722 (2024)].Biomol Ther (Seoul). 2025 Nov 1;33(6):1085. doi: 10.4062/biomolther.2025.007. Biomol Ther (Seoul). 2025. PMID: 41169260 Free PMC article. No abstract available.
Abstract
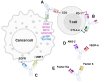
Bispecific antibodies represent a significant advancement in therapeutic antibody engineering, offering the ability to simultaneously target two distinct antigens. This dual-targeting capability enhances therapeutic efficacy, especially in complex diseases, such as cancer and autoimmune disorders, where drug resistance and incomplete target coverage are prevalent challenges. Bispecific antibodies facilitate immune cell engagement and disrupt multiple signaling pathways, providing a more comprehensive treatment approach than traditional monoclonal antibodies. However, the intricate structure of bispecific antibodies introduces unique pharmacokinetic challenges, including issues related to their absorption, distribution, metabolism, and excretion, which can significantly affect their efficacy and safety. This review provides an in-depth analysis of the structural design, mechanisms of action, and pharmacokinetics of the currently approved bispecific antibodies. It also highlights the engineering innovations that have been implemented to overcome these challenges, such as Fc modifications and advanced dimerization techniques, which enhance the stability and half-life of bispecific antibodies. Significant progress has been made in bispecific antibody technology; however, further research is necessary to broaden their clinical applications, enhance their safety profiles, and optimize their incorporation into combination therapies. Continuous advancements in this field are expected to enable bispecific antibodies to provide more precise and effective therapeutic strategies for a range of complex diseases, ultimately improving patient outcomes and advancing precision medicine.
Keywords: Antibody engineering; Bispecific antibody; Dual-targeting therapy; Immunotherapy; Mechanism of action; Pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ahmed H., Mahmud A. R., Siddiquee M. F., Shahriar A., Biswas P., Shimul M. E. K., Ahmed S. Z., Ema T. I., Rahman N., Khan M. A., Mizan M. F. R., Emran T. B. Role of T cells in cancer immunotherapy: opportunities and challenges. Cancer Pathog. Ther. 2023;1:116–126. doi: 10.1016/j.cpt.2022.12.002. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

