Human TRPV1 is an efficient thermogenetic actuator for chronic neuromodulation
- PMID: 39448456
- PMCID: PMC11502623
- DOI: 10.1007/s00018-024-05475-x
Human TRPV1 is an efficient thermogenetic actuator for chronic neuromodulation
Abstract
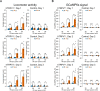
Thermogenetics is a promising neuromodulation technique based on the use of heat-sensitive ion channels. However, on the way to its clinical application, a number of questions have to be addressed. First, to avoid immune response in future human applications, human ion channels should be studied as thermogenetic actuators. Second, heating levels necessary to activate these channels in vivo in brain tissue should be studied and cytotoxicity of these temperatures addressed. Third, the possibility and safety of chronic neuromodulation has to be demonstrated. In this study, we present a comprehensive framework for thermogenetic neuromodulation in vivo using the thermosensitive human ion channel hTRPV1. By targeting hTRPV1 expression to excitatory neurons of the mouse brain and activating them within a non-harmful temperature range with a fiber-coupled infrared laser, we not only induced neuronal firing and stimulated locomotion in mice, but also demonstrated that thermogenetics can be employed for repeated neuromodulation without causing evident brain tissue injury. Our results lay the foundation for the use of thermogenetic neuromodulation in brain research and therapy of neuropathologies.
Keywords: Calcium imaging; Neuromodulation; Neuronal activity; TRP channels; TRPV1; Thermogenetics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Boyden ES, Zhang F, Bamberg E et al (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8:1263–1268. 10.1038/nn1525 - PubMed
-
- Zhang F, Wang L-P, Brauner M et al (2007) Multimodal fast optical interrogation of neural circuitry. Nature 446:633–639. 10.1038/nature05744 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

