Long-term effects of a double hit murine model for schizophrenia on parvalbumin expressing cells and plasticity-related molecules in the thalamic reticular nucleus and the habenula
- PMID: 39448557
- PMCID: PMC11502763
- DOI: 10.1038/s41398-024-03166-6
Long-term effects of a double hit murine model for schizophrenia on parvalbumin expressing cells and plasticity-related molecules in the thalamic reticular nucleus and the habenula
Abstract
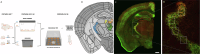
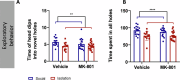
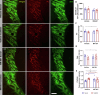
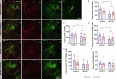
The exposure to aversive experiences during early-life affects brain maturation and induces changes in behavior. Additionally, when these experiences coincide with subtle neurodevelopmental alterations, they may contribute to the emergence of psychiatric disorders, such as schizophrenia. Studies in patients and animal models have identified changes in parvalbumin (PV) expressing inhibitory neurons, highlighting their significance in the etiology of this disorder. Most studies have been focused on the cortex, but PV+ neurons also provide inhibitory input to diencephalic regions, particularly to the thalamus (through cells in the thalamic reticular nucleus, TRN) and the habenula. Remarkably, alterations in both nuclei have been described in schizophrenia. Some of these changes in PV+ cells may be mediated by perineuronal nets (PNN), specialized regions of the extracellular matrix that often surround them and regulate their synaptic input and activity. Interestingly, the physiological maturation and integration of PV+ neurons, which involves the assembly of PNN, occurs during early postnatal life. Plasticity molecules associated to inhibitory neurons, such as PSA-NCAM, or NMDA receptors (NMDAR) can also influence the structure and function of these cells. Growing evidence also indicates that glial cells regulate the physiology of PV+ neurons by influencing their maturation and modulating their synaptic connectivity. To explore the impact of early-life aversive experiences and concomitant subtle neurodevelopmental alterations on diencephalic PV+ cells, we analyzed adult male mice subjected to a double-hit model (DHM) of schizophrenia, combining a single injection of an NMDAR antagonist at P7 and post-weaning social isolation. We observed that exploratory behavior, PV+ neurons and their associated PNN, as well as PSA-NCAM and NMDAR expression and glial cells, in the TRN and the habenula were affected by the DHM or one of its factors. To our knowledge, this is the first report on such alterations in these diencephalic structures in an animal model combining neurodevelopmental alterations and early-life stress during adolescence. Our findings complement previous work on PV+ neurons in cortical regions and underscore the importance of studying diencephalic inhibitory networks and their intricate interactions with aversive experiences and neurodevelopmental alterations during early life in the context of schizophrenia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

