Precise genetic control of ATOH1 enhances maturation of regenerated hair cells in the mature mouse utricle
- PMID: 39448563
- PMCID: PMC11502789
- DOI: 10.1038/s41467-024-53153-0
Precise genetic control of ATOH1 enhances maturation of regenerated hair cells in the mature mouse utricle
Abstract
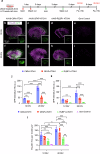
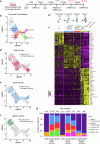
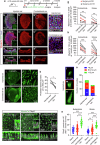
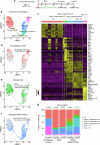
Vestibular hair cells are mechanoreceptors critical for detecting head position and motion. In mammals, hair cell loss causes vestibular dysfunction as spontaneous regeneration is nearly absent. Constitutive expression of exogenous ATOH1, a hair cell transcription factor, increases hair cell regeneration, however, these cells fail to fully mature. Here, we profiled mouse utricles at 14 time points, and defined transcriptomes of developing and mature vestibular hair cells. To mimic native hair cells which downregulate endogenous ATOH1 as they mature, we engineered viral vectors carrying the supporting cell promoters GFAP and RLBP1. In utricles damaged ex vivo, both CMV-ATOH1 and GFAP-ATOH1 increased regeneration more effectively than RLBP1-ATOH1, while GFAP-ATOH1 and RLBP1-ATOH1 induced hair cells with more mature transcriptomes. In utricles damaged in vivo, GFAP-ATOH1 induced regeneration of hair cells expressing genes indicative of maturing type II hair cells, and more hair cells with bundles and synapses than untreated organs. Together our results demonstrate the efficacy of spatiotemporal control of ATOH1 overexpression in inner ear hair cell regeneration.
© 2024. The Author(s).
Conflict of interest statement
T.Y., A.K., G.P., R.M., B.H., X.W., L.B., N.P., K.S., S.N., M.K., T.G., N.D., and J.B. were employees of Decibel Therapeutics. G.P., R.M., L.B., N.P., and K.S. are current employees of Regeneron Pharmaceuticals. A.G.C. was a member of Decibel Therapeutics’ scientific advisory board. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 DC021110/DC/NIDCD NIH HHS/United States
- R01DC016919/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
- R01 DC016919/DC/NIDCD NIH HHS/United States
- K24 DC020986/DC/NIDCD NIH HHS/United States
- R01 DC020879/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

