Supercharged fluorescent proteins detect lanthanides via direct antennae signaling
- PMID: 39448572
- PMCID: PMC11502933
- DOI: 10.1038/s41467-024-53106-7
Supercharged fluorescent proteins detect lanthanides via direct antennae signaling
Abstract
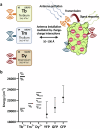
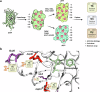
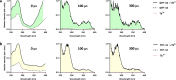
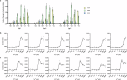
A sustainable operation for harvesting metals in the lanthanide series is needed to meet the rising demand for rare earth elements across diverse global industries. However, existing methods are limited in their capacity for detection and capture at environmentally and industrially relevant lanthanide concentrations. Supercharged fluorescent proteins have solvent-exposed, negatively charged residues that potentially create multiple direct chelation pockets for free lanthanide cations. Here, we demonstrate that negatively supercharged proteins can bind and quantitatively report concentrations of lanthanides via an underutilized lanthanide-to-chromophore pathway of energy transfer. The top-performing sensors detect lanthanides in the micromolar to millimolar range and remain unperturbed by environmentally significant concentrations of competing metals. As a demonstration of the versatility and adaptability of this energy transfer method, we show proximity and signal transmission between the lanthanides and a supramolecular assembly of supercharged proteins, paving the way for the detection of lanthanides via programmable protein oligomers and materials.
© 2024. The Author(s).
Conflict of interest statement
K.Y.H., A.D.E., and D.J.F.W. are co-inventors on the U.S. patent application pertaining to the use of supercharged proteins for lanthanide detection (Application No. 63/611,409). Applicant: Board of Regents, The University of Texas System. The remaining authors declare no competing interests.
Figures

References
-
- Eliseeva, S. V. & Bünzli, J.-C. G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev.39, 189–227 (2010). - PubMed
-
- Woodruff, D. N., Winpenny, R. E. P. & Layfield, R. A. Lanthanide single-molecule magnets. Chem. Rev.113, 5110–5148 (2013). - PubMed
-
- Edelmann, F. T. Lanthanide amidinates and guanidinates in catalysis and materials science: a continuing success story. Chem. Soc. Rev.41, 7657 (2012). - PubMed
-
- Wall, F. Rare earth elements. Encyclopedia of Geology 680–693 (Elsevier, 2021). 10.1016/B978-0-08-102908-4.00101-6.
-
- Lucas, J., Lucas, P., Le Mercier, T., Rollat, A. & Davenport, W. G. Rare Earths: Science, Technology, Production and Use. (Elsevier, 2015).
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- W912HZ-22-Q-0016/United States Department of Defense | United States Army | US Army Corps of Engineers | Engineer Research and Development Center (U.S. Army Engineer Research and Development Center)
- W912HZ-22-2-0037/United States Department of Defense | United States Army | US Army Corps of Engineers | Engineer Research and Development Center (U.S. Army Engineer Research and Development Center)
- W912HZ-22-2-0037/United States Department of Defense | United States Army | US Army Corps of Engineers | Engineer Research and Development Center (U.S. Army Engineer Research and Development Center)
- W911NF2220246/United States Department of Defense | United States Army | U.S. Army Research, Development and Engineering Command | Army Research Office (ARO)
- W911NF2320089/United States Department of Defense | United States Army | U.S. Army Research, Development and Engineering Command | Army Research Office (ARO)
LinkOut - more resources
Full Text Sources
Research Materials

