Osteopontin is a therapeutic target that drives breast cancer recurrence
- PMID: 39448577
- PMCID: PMC11502809
- DOI: 10.1038/s41467-024-53023-9
Osteopontin is a therapeutic target that drives breast cancer recurrence
Abstract
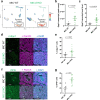
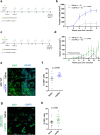
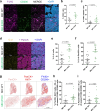
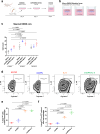
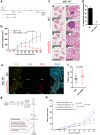
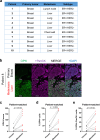
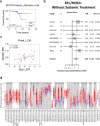
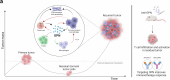
Recurrent breast cancers often develop resistance to standard-of-care therapies. Identifying targetable factors contributing to cancer recurrence remains the rate-limiting step in improving long-term outcomes. In this study, we identify tumor cell-derived osteopontin as an autocrine and paracrine driver of tumor recurrence. Osteopontin promotes tumor cell proliferation, recruits macrophages, and synergizes with IL-4 to further polarize them into a pro-tumorigenic state. Macrophage depletion and osteopontin inhibition decrease recurrent tumor growth. Furthermore, targeting osteopontin in primary tumor-bearing female mice prevents metastasis, permits T cell infiltration and activation, and improves anti-PD-1 immunotherapy response. Clinically, osteopontin expression is higher in recurrent metastatic tumors versus female patient-matched primary breast tumors. Osteopontin positively correlates with macrophage infiltration, increases with higher tumor grade, and its elevated pathway activity is associated with poor prognosis and long-term recurrence. Our findings suggest clinical implications and an alternative therapeutic strategy based on osteopontin's multiaxial role in breast cancer progression and recurrence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Gu, Y., Bui, T. & Muller, W. J. Exploiting mouse models to recapitulate clinical tumor dormancy and recurrence in breast cancer. Endocrinology163, bqac055 (2022). - PubMed
-
- Goddard, E. T., Bozic, I., Riddell, S. R. & Ghajar, C. M. Dormant tumour cells, their niches and the influence of immunity. Nat. Cell Biol.20, 1240–1249 (2018). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
- FDN-148373/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- PLL - 190347/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- 187660/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- 706679/Canadian Cancer Society Research Institute (Société Canadienne du Cancer)
- 706216/Canadian Cancer Society Research Institute (Société Canadienne du Cancer)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

