Chronic hypoxia for the adaptation of extracellular vesicle phenotype
- PMID: 39448620
- PMCID: PMC11502752
- DOI: 10.1038/s41598-024-73453-1
Chronic hypoxia for the adaptation of extracellular vesicle phenotype
Abstract
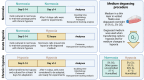
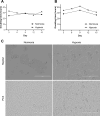
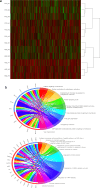
Variations in oxygen level affect the phenotype of cells and extracellular vesicles (EVs). Depending on the metabolic oxygen demand of cells, hypoxic cell culture can produce conditions more like those found in vivo, and with appropriate oxygen levels, mimic hypoxic tumours. However, most previous experiments studying both EVs and the effects of hypoxia on cells use periods of 72 h or less of hypoxia. We hypothesised that this was insufficient time for adaptation to hypoxic conditions both for EVs and cells which may skew the results of such studies. In this study, the effects of acute (72 h) and chronic hypoxia (> 2 weeks) on the phenotype of HepG2 and PC3 cells and their EVs were examined. Cells could be cultured normally under chronic hypoxic conditions and cryopreserved and recovered. The effects of hypoxia on EV phenotype are slow to establish and dependent on cell line. In PC3 cells, the greatest change in phenotype and increase in EV production occurred only with chronic hypoxic culture. In HepG2 cells, the number of EVs produced was insensitive to hypoxic culture and the greatest changes in protein expression were observed after acute hypoxic culture. Nonetheless, biphasic changes in EV phenotype were detected in both cell types in response to either acute or chronic hypoxia. These results indicate that for cells which do not induce consumptive oxygen depletion, prolonged hypoxic culture is required for complete adaptation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bell, C. C. et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep [Internet]. May 4 [cited 2016 Dec 8];6(1):25187. (2016). http://www.nature.com/articles/srep25187 - PMC - PubMed
-
- Place, T. L., Domann, F. E. & Case, A. J. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic Biol Med [Internet]. ;113:311–22. (2017). https://linkinghub.elsevier.com/retrieve/pii/S0891584917307864 - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 337430/Academy of Finland GeneCellNano Flagship
- 337430/Academy of Finland GeneCellNano Flagship
- 337430/Academy of Finland GeneCellNano Flagship
- 337430/Academy of Finland GeneCellNano Flagship
- 337430/Academy of Finland GeneCellNano Flagship
- 337430/Academy of Finland GeneCellNano Flagship
- 114339/Blood Service Research Foundation
- 114339/Blood Service Research Foundation
- 114339/Blood Service Research Foundation
- 315409/Research Council of Finland
- 315409/Research Council of Finland
- 1842/312019/EVE ecosystem-project
- 1842/312019/EVE ecosystem-project
- 00220283/Suomen Kulttuurirahasto
LinkOut - more resources
Full Text Sources

