Structure, interaction and nervous connectivity of beta cell primary cilia
- PMID: 39448638
- PMCID: PMC11502866
- DOI: 10.1038/s41467-024-53348-5
Structure, interaction and nervous connectivity of beta cell primary cilia
Abstract
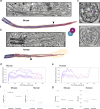
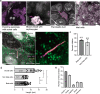
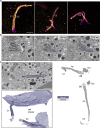
Primary cilia are sensory organelles present in many cell types, partaking in various signaling processes. Primary cilia of pancreatic beta cells play pivotal roles in paracrine signaling and their dysfunction is linked to diabetes. Yet, the structural basis for their functions is unclear. We present three-dimensional reconstructions of beta cell primary cilia by electron and expansion microscopy. These cilia are spatially confined within deep ciliary pockets or narrow spaces between cells, lack motility components and display an unstructured axoneme organization. Furthermore, we observe a plethora of beta cell cilia-cilia and cilia-cell interactions with other islet and non-islet cells. Most remarkably, we have identified and characterized axo-ciliary synapses between beta cell cilia and the cholinergic islet innervation. These findings highlight the beta cell cilia's role in islet connectivity, pointing at their function in integrating islet intrinsic and extrinsic signals and contribute to understanding their significance in health and diabetes.
© 2024. The Author(s).
Conflict of interest statement
C.S.X. and H.F.H. are the inventors of a US patent assigned to HHMI for the enhanced FIB-SEM systems used in this work: Xu, C.S., Hayworth, K.J., and Hess, H.F. (2020) Enhanced FIB-SEM systems for large-volume 3D imaging. US Patent 10,600,615, 24 Mar. 2020. The other authors declare no competing interests.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

