Versatile gamma-tubulin complexes contribute to the dynamic organization of MTOCs during Drosophila spermatogenesis
- PMID: 39448788
- PMCID: PMC11502891
- DOI: 10.1038/s42003-024-07090-9
Versatile gamma-tubulin complexes contribute to the dynamic organization of MTOCs during Drosophila spermatogenesis
Abstract
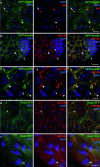
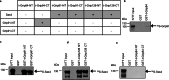
The initiation of microtubule formation is facilitated by γ-tubulin and γ-Tubulin Ring Complex (γ-TuRC) in various microtubule-organizing centers (MTOCs). While the heterogeneity of tissue-specific MTOCs and γ-TuRC in Drosophila testis has been described, their molecular composition and physiological significance are poorly understood. We investigated the testis-specific distribution and biochemical interaction of the canonical γ-TuRC proteins Grip163 and Grip84. We found that while Grip163 is present on the centrosome and basal body, Grip84 localizes to the centrosome and Golgi in spermatocytes and colocalizes with the testis-specific γ-Tubulin complexes (t-γ-TuC) at the basal body, apical nuclear tip, and near the elongated mitochondria after meiosis. We also showed the apical nuclear tip localization of some γ-TuRC interacting partners and proved their binding to t-γ-TuC proteins. These results highlight and prove the importance of the different γ-TuRCs in organizing the diverse MTOCs present during the extensive rearrangement of cell organelles during the spermatogenesis of Drosophila.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

