Subclassification of obesity for precision prediction of cardiometabolic diseases
- PMID: 39448862
- PMCID: PMC11835733
- DOI: 10.1038/s41591-024-03299-7
Subclassification of obesity for precision prediction of cardiometabolic diseases
Erratum in
-
Author Correction: Subclassification of obesity for precision prediction of cardiometabolic diseases.Nat Med. 2025 Feb;31(2):695. doi: 10.1038/s41591-024-03403-x. Nat Med. 2025. PMID: 39572748 Free PMC article. No abstract available.
Abstract
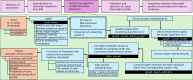
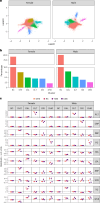
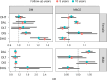
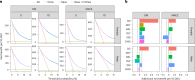
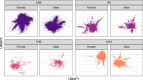
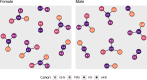
Obesity and cardiometabolic disease often, but not always, coincide. Distinguishing subpopulations within which cardiometabolic risk diverges from the risk expected for a given body mass index (BMI) may facilitate precision prevention of cardiometabolic diseases. Accordingly, we performed unsupervised clustering in four European population-based cohorts (N ≈ 173,000). We detected five discordant profiles consisting of individuals with cardiometabolic biomarkers higher or lower than expected given their BMI, which generally increases disease risk, in total representing ~20% of the total population. Persons with discordant profiles differed from concordant individuals in prevalence and future risk of major adverse cardiovascular events (MACE) and type 2 diabetes. Subtle BMI-discordances in biomarkers affected disease risk. For instance, a 10% higher probability of having a discordant lipid profile was associated with a 5% higher risk of MACE (hazard ratio in women 1.05, 95% confidence interval 1.03, 1.06, P = 4.19 × 10-10; hazard ratio in men 1.05, 95% confidence interval 1.04, 1.06, P = 9.33 × 10-14). Multivariate prediction models for MACE and type 2 diabetes performed better when incorporating discordant profile information (likelihood ratio test P < 0.001). This enhancement represents an additional net benefit of 4-15 additional correct interventions and 37-135 additional unnecessary interventions correctly avoided for every 10,000 individuals tested.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: T.S. and C.D. are employees and shareholders of Novo Nordisk. M.F.G. has received financial and nonfinancial (in kind) support from Boehringer Ingelheim Pharma GmbH, JDRF International, Eli Lilly, AbbVie, Sanofi-Aventis, Astellas, Novo Nordisk A/S and Bayer AG, within European Union grant H2020-JTI-lMl2-2015-05 (grant agreement no. 115974 - BEAt-DKD). She has also received financial and in kind support from Novo Nordisk, Pfizer, Follicum, Coegin Pharma, Abcentra, Probi and Johnson & Johnson, within a project funded by the Swedish Foundation for Strategic Research on precision medicine in diabetes (LUDC-IRC, grant no. 15-0067). M.F.G. has received personal consultancy fees from Lilly and Tribune Therapeutics AB. M.P.-N. is an employee of Boehringer Ingelheim. Outside the submitted work, P.S.W. has received consulting fees from Astra Zeneca, research funding from Bayer AG, research funding, consulting and lecturing fees from Bayer Health Care, lecturing fees from Bristol Myers Squibb, research funding and consulting fees from Boehringer Ingelheim, research funding and consulting fees from Daiichi Sankyo Europe, consulting fees and nonfinancial support from Diasorin, nonfinancial research support from I.E.M., research funding and consulting fees from Novartis Pharma, lecturing fees from Pfizer Pharma, nonfinancial grants from Philips Medical Systems, and research funding and consulting fees from Sanofi-Aventis. C.L.R. reports grants from the Irish Research Council, Science Foundation Ireland, Anabio and the Health Research Board. He serves on advisory boards and speakers panels of Novo Nordisk, Roche, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Glia, Irish Life Health, Boehringer Ingelheim, Currax, Zealand Pharma, Keyron, Astra Zeneca and Rhythm Pharma. C.L.R. is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. C.L.R. provides obesity clinical care in the My Best Weight clinic and Beyond BMI clinic and is a shareholder in these clinics. He was the chief medical officer and director of the Medical Device Division of Keyron in 2021. Both of these are unremunerated positions. C.L.R. was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. No patients have been included in any of Keyron’s studies and they are not listed on the stock market. C.L.R. was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. Outside the submitted work, E.R.P. has received honoraria from Novo Nordisk, Lilly and Illumina. Within the past five years, P.W.F. has received consulting honoraria from Eli Lilly Inc., Novo Nordisk Foundation, Novo Nordisk A/S, UBS and Zoe Ltd, has been an employee of the Novo Nordisk Foundation, and has been on advisory boards for the Danish Diabetes and Endocrine Academy, Novo Nordisk A/S, Hamad Medical Corporation and Zoe Ltd. P.W.F. has also received investigator-initiated grants (paid to institution) from numerous pharmaceutical companies as part of the Innovative Medicines Initiative of the European Union. The other authors declare no competing interests.
Figures




References
-
- World Obesity Atlas 2023. World Obesitydata.worldobesity.org/publications/?cat=19 (2023).
-
- Gradmark, A. M. I. et al. Computed tomography-based validation of abdominal adiposity measurements from ultrasonography, dual-energy X-ray absorptiometry and anthropometry. Br. J. Nutr.104, 582–588 (2010). - PubMed
-
- Prillaman, M. Why BMI is flawed — and how to redefine obesity. Nature622, 232–233 (2023). - PubMed
-
- Rothman, K. J. BMI-related errors in the measurement of obesity. Int. J. Obes.32, S56–S59 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

