RIP1 inhibition protects retinal ganglion cells in glaucoma models of ocular injury
- PMID: 39448868
- PMCID: PMC11802773
- DOI: 10.1038/s41418-024-01390-7
RIP1 inhibition protects retinal ganglion cells in glaucoma models of ocular injury
Abstract
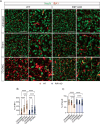
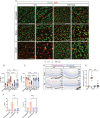
Receptor-interacting protein 1 (RIP1, RIPK1) is a critical mediator of multiple signaling pathways that promote inflammatory responses and cell death. The kinase activity of RIP1 contributes to the pathogenesis of a number of inflammatory and neurodegenerative diseases. However, the role of RIP1 in retinopathies remains unclear. This study demonstrates that RIP1 inhibition protects retinal ganglion cells (RGCs) in preclinical glaucoma models. Genetic inactivation of RIP1 improves RGC survival and preserves retinal function in the preclinical glaucoma models of optic nerve crush (ONC) and ischemia-reperfusion injury (IRI). In addition, the involvement of necroptosis in ONC and IRI glaucoma models was examined by utilizing RIP1 kinase-dead (RIP1-KD), RIP3 knockout (RIP3-KO), and MLKL knockout (MLKL-KO) mice. The number of RGCs, retinal thickness, and visual acuity were rescued in RIP1-kinase-dead (RIP1-KD) mice in both models, while wild-type (WT) mice experienced significant retinal thinning, RGC loss, and vision impairment. RIP3-KO and MLKL-KO mice showed moderate protective effects in the IRI model and limited in the ONC model. Furthermore, we confirmed that a glaucoma causative mutation in optineurin, OPTN-E50K, sensitizes cells to RIP1-mediated inflammatory cell death. RIP1 inhibition reduces RGC death and axonal degeneration following IRI in mice expressing OPTN-WT and OPTN-E50K variant mice. We demonstrate that RIP1 inactivation suppressed microglial infiltration in the RGC layer following glaucomatous damage. Finally, this study highlights that human glaucomatous retinas exhibit elevated levels of TNF and RIP3 mRNA and microglia infiltration, thus demonstrating the role of neuroinflammation in glaucoma pathogenesis. Altogether, these data indicate that RIP1 plays an important role in modulating neuroinflammation and that inhibiting RIP1 activity may provide a neuroprotective therapy for glaucoma.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: This study is supported by F. Hoffmann-La Roche Ltd. BKK, SA, FR, and PDW are employees of F. Hoffmann-La Roche. DV, JDW, and TG are employees of Genentech Inc. The authors declare no competing interests. Ethics approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations. All experimental animal studies with mice were conducted under the approval of the Institutional Animal Care and Use Committees of Genentech Lab Animal Research. For human samples, informed consent was obtained from all participants.
Figures





References
-
- Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. - PubMed
-
- Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390:2183–93. - PubMed
-
- Lundberg L, Wettrell K, Linnér E. Ocular hypertension. A prospective twenty-year follow-up study. Acta Ophthalmol. 1987;65:705–8. - PubMed
-
- Kitazawa Y, Horie T, Aoki S, Suzuki M, Nishioka K. Untreated ocular hypertension. A long-term prospective study. Arch Ophthalmol. 1977;95:1180–4. - PubMed
-
- Gupta N, Weinreb RN. New definitions of glaucoma. Curr Opin Ophthalmol. 1997;8:38–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

