The impact of HER2-low status on pathological complete response and disease-free survival in early-stage breast cancer
- PMID: 39448928
- PMCID: PMC11515358
- DOI: 10.1186/s12885-024-13064-1
The impact of HER2-low status on pathological complete response and disease-free survival in early-stage breast cancer
Abstract
Background: The HER-2 status of breast cancer (BC) has been classified as negative or positive for a long time. Given the efficacy of novel anti-HER2-targeted antibody drug conjugates (ADCs) in HER2-low BC, a distinct subgroup of HER2-low tumors has emerged within BC. The biology and prognostic impact of HER2-low expression are not yet well defined, and inconsistent results were reported. This study aims to evaluate the impact of low HER-2 status on the response to neoadjuvant chemotherapy (NACT) and disease- free survival (DFS) rates.
Methods: We retrospectively analyzed BC patients treated with NACT from 2017 to 2023 in two cancer centers. HER2-negative patients were included. HER-2 low status was defined by IHC + 1 or + 2/ISH non-amplified, and HER2-zero was defined by IHC 0. Pathological complete response (pCR) rates and DFS between HER2-low and HER2-zero populations were compared.
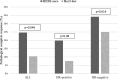
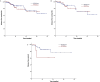
Results: 170 patients were identified. 122 (72%) of these patients were HER2- zero BC, whereas 48 (28%) were HER2-low BC. Overall, pCR was achieved in 35 (20.5%) patients. Of these, pCR was observed in 30 patients (44.6%) from the HER2- zero group, compared to 5 patients (10.4%) from the HER2-low group (p = 0.046), but significance was lost in multivariate analysis. Among the hormone receptor (HR) positive subtype, pCR was achieved 19.8% of HER2-zero tumors and 7.5% of HER2-low tumors (p = 0.08). For HR-negative subtype 34.1% HER2-zero tumors had pCR and 25% of the HER2-low tumors had pCR (p = 0.614). There was no association between DFS and HER2-low status.
Conclusions: Our study indicates that HER2-low status had no impact on pCR or DFS.
Keywords: Breast cancer; HER2-low expression; Pathological complete response.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous