Risk of Clostridioides difficile infection in inflammatory bowel disease patients undergoing vedolizumab treatment: a systematic review and meta-analysis
- PMID: 39448963
- PMCID: PMC11515399
- DOI: 10.1186/s12876-024-03460-z
Risk of Clostridioides difficile infection in inflammatory bowel disease patients undergoing vedolizumab treatment: a systematic review and meta-analysis
Abstract
Background: Inflammatory bowel disease (IBD) is a chronic, relapsing condition wherein biologics have improved disease prognosis but introduced elevated infection susceptibility. Vedolizumab (VDZ) demonstrates unique safety advantages; however, a comprehensive systematic comparison regarding the risk of Clostridioides difficile infection (CDI) between vedolizumab and alternative medications remains absent.
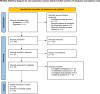
Method: Medline, Embase, Cochrane, and clinicaltrials.gov registry were comprehensively searched. Pooled estimates of CDI proportion, incidence, pooled risk ratio between ulcerative colitis (UC) and Crohn's disease (CD), vedolizumab and other medications were calculated. Data synthesis was completed in R using the package "meta".
Results: Of the 338 studies initially identified, 30 met the inclusion/exclusion criteria. For CDI risk, the pooled proportion was 0.013 (95% CI 0.010-0.017), as well as the pooled proportion of serious CDI was 0.004 (95% CI 0.002-0.008). The comparative pooled risk ratios revealed: UC versus CD at 2.25 (95% CI 1.73-2.92), vedolizumab versus anti-TNF agents at 0.15 (95% CI 0.04-0.63) for UC and 1.29 (95% CI 0.41-4.04) for CD.
Conclusion: The overall CDI risk in IBD patients exposed to vedolizumab was estimated to be 0.013. An increased risk of CDI was noted in UC patients receiving vedolizumab compared to those with CD. Vedolizumab potentially offers an advantage over anti-TNF agents for UC regarding CDI risk, but not for CD.
Trial registration: The study was registered on the PROSPERO registry (CRD42023465986).
Keywords: Clostridioides difficile infection; Inflammatory bowel disease; Systematic review; Vedolizumab.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

