Gut-first Parkinson's disease is encoded by gut dysbiome
- PMID: 39449004
- PMCID: PMC11515425
- DOI: 10.1186/s13024-024-00766-0
Gut-first Parkinson's disease is encoded by gut dysbiome
Abstract
Background: In Parkinson's patients, intestinal dysbiosis can occur years before clinical diagnosis, implicating the gut and its microbiota in the disease. Recent evidence suggests the gut microbiota may trigger body-first Parkinson Disease (PD), yet the underlying mechanisms remain unclear. This study aims to elucidate how a dysbiotic microbiome through intestinal immune alterations triggers PD-related neurodegeneration.
Methods: To determine the impact of gut dysbiosis on the development and progression of PD pathology, wild-type male C57BL/6 mice were transplanted with fecal material from PD patients and age-matched healthy donors to challenge the gut-immune-brain axis.
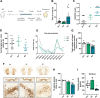
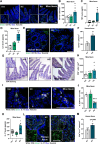
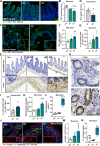
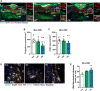
Results: This study demonstrates that patient-derived intestinal microbiota caused midbrain tyrosine hydroxylase positive (TH +) cell loss and motor dysfunction. Ileum-associated microbiota remodeling correlates with a decrease in Th17 homeostatic cells. This event led to an increase in gut inflammation and intestinal barrier disruption. In this regard, we found a decrease in CD4 + cells and an increase in pro-inflammatory cytokines in the blood of PD transplanted mice that could contribute to an increase in the permeabilization of the blood-brain-barrier, observed by an increase in mesencephalic Ig-G-positive microvascular leaks and by an increase of mesencephalic IL-17 levels, compatible with systemic inflammation. Furthermore, alpha-synuclein aggregates can spread caudo-rostrally, causing fragmentation of neuronal mitochondria. This mitochondrial damage subsequently activates innate immune responses in neurons and triggers microglial activation.
Conclusions: We propose that the dysbiotic gut microbiome (dysbiome) in PD can disrupt a healthy microbiome and Th17 homeostatic immunity in the ileum mucosa, leading to a cascade effect that propagates to the brain, ultimately contributing to PD pathophysiology. Our landmark study has successfully identified new peripheral biomarkers that could be used to develop highly effective strategies to prevent the progression of PD into the brain.
Keywords: Dopaminergic; Dorsal motor nucleus of the vagus; Dysbiome; Dysbiosis; Gut microbiome; Gut-brain axis; Inflammation; Innate immunity; Mitochondria; Parkinson’s disease; Substantia nigra.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures




References
-
- Lin C-H, Chen C-C, Chiang H-L, Liou J-M, Chang C-M, Lu T-P, et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation. 2019;16:129. Available from: https://pubmed.ncbi.nlm.nih.gov/31248424. - PMC - PubMed
-
- Esteves AR, Munoz-Pinto MF, Nunes-Costa D, Candeias E, Silva DF, Magalhães JD, et al. Footprints of a microbial toxin from the gut microbiome to mesencephalic mitochondria. Gut. 2023;72:73. Available from: http://gut.bmj.com/content/72/1/73.abstract. - PMC - PubMed
-
- Braak H, Sastre M, Bohl JRE, de Vos RAI, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113:421–9. Available from: 10.1007/s00401-007-0193-x. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

