Blautia coccoides and its metabolic products enhance the efficacy of bladder cancer immunotherapy by promoting CD8+ T cell infiltration
- PMID: 39449013
- PMCID: PMC11515615
- DOI: 10.1186/s12967-024-05762-y
Blautia coccoides and its metabolic products enhance the efficacy of bladder cancer immunotherapy by promoting CD8+ T cell infiltration
Abstract
Background: Immune checkpoint inhibitors (ICIs) have emerged as a novel and effective treatment strategy, yet their effectiveness is limited to a subset of patients. The gut microbiota, recognized as a promising anticancer adjuvant, is being increasingly suggested to augment the efficacy of ICIs. Despite this, the causal link between the gut microbiota and the success of immunotherapy is not well understood. This gap in knowledge has driven us to identify beneficial microbiota and explore the underlying molecular mechanisms.
Methods: Through 16S rDNA sequencing, we identified distinct gut microbiota in patients undergoing treatment with ICIs. Following this, we assessed the impact of probiotics on anti-PD-1 therapy in bladder cancer using mouse models, employing a multi-omics strategy. Subsequently, we uncovered the mechanisms through which Blautia-produced metabolites enhance antitumor immunity, utilizing untargeted metabolomics and a range of molecular biology techniques.
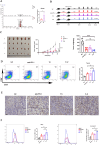
Results: In our research, the LEfSe analysis revealed a significant enrichment of the Blautia genus in the gut microbiota of patients who responded to immunotherapy. We discovered that the external addition of Blautia coccoides hampers tumor growth in a bladder cancer mouse model by enhancing the infiltration of CD8+ T cells within the tumor microenvironment (TME). Further investigations through untargeted metabolomics and molecular biology experiments showed that oral administration of Blautia coccoides elevated trigonelline levels. This, in turn, suppresses the β-catenin expression both in vitro and in vivo, thereby augmenting the cancer-killing activity of CD8+ T cells.
Conclusions: This research provided valuable insights into enhancing the efficacy of PD-1 inhibitors in clinical settings. It was suggested that applying Blautia coccoides and its metabolic product, trigonelline, could serve as a synergistic treatment method with PD-1 inhibitors in clinical applications.
Keywords: Blautia coccoides; Anti-PD-1 immunotherapy; Gut microbiota; Trigonelline.
© 2024. The Author(s).
Conflict of interest statement
None declared.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

