Safety and adherence of early oral immunotherapy for peanut allergy in a primary care setting: a retrospective cross-sectional study
- PMID: 39449085
- PMCID: PMC11515316
- DOI: 10.1186/s13223-024-00916-5
Safety and adherence of early oral immunotherapy for peanut allergy in a primary care setting: a retrospective cross-sectional study
Abstract
Background: Peanut allergy is a common food allergy with potentially life-threatening implications. Early oral immunotherapy for peanut allergy (P-EOIT) has been shown to be effective and safe in research and specialty clinic settings. Provision of P-EOIT in primary care would make it available to more patients. We sought to assess the safety of P-EOIT in a primary care setting by documenting the rates of peanut-related allergic reactions leading to emergency department (ED) visits and use of epinephrine. We also examined adherence by assessing the percentage of patients reaching maintenance phase and continuing ingestion after one year of P-EOIT.
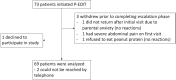
Methods: This retrospective study included all patients aged less than 36 months who started P-EOIT at a primary care allergy clinic in New Brunswick, Canada, from 2016 to 2020. The population included patients who (1) had a history of an allergic reaction to peanuts with a positive skin prick test or positive peanut specific IgE level (ps-IgE) or (2) no history of ingestion and a baseline ps-IgE ≥5 kU/L. Patients had biweekly clinic visits with graded increases in peanut protein up to a maintenance dose of 300 mg of peanut protein daily. A blinded retrospective review of paper charts and electronic medical records was conducted along with phone interviews regarding ED visits and epinephrine use.
Results: All 69 consented patients reached maintenance dose over a median of 29 weeks, and 66 patients (95.7%) were still regularly consuming peanut protein after 1 year of maintenance. One patient had a peanut ingestion-related ED visit requiring epinephrine during the escalation phase of peanut protein dosing (1.4%). During the first year of maintenance phase, no patients had peanut ingestion-related ED visits nor required epinephrine.
Conclusion: Early oral immunotherapy for peanut allergy in a primary care setting appears to be safe and our findings suggest that it does not lead to an increased burden of emergency department visits. Our population had high adherence rates, with the majority achieving maintenance dose and staying on this dose for one year.
Keywords: Oral immunotherapy; Peanut allergy; Preschool; Primary care.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
References
LinkOut - more resources
Full Text Sources