Harnessing the power of patient engagement in evaluating a novel brace for knee osteoarthritis: a co-produced commentary
- PMID: 39449106
- PMCID: PMC11515838
- DOI: 10.1186/s40900-024-00640-9
Harnessing the power of patient engagement in evaluating a novel brace for knee osteoarthritis: a co-produced commentary
Abstract
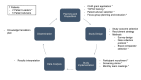
Introduction: Patient oriented research (POR) invites patients to partner with researchers, clinicians, and other stakeholders, incorporating diverse perspectives to generate scientific evidence meaningful to all parties involved. We adopted a POR approach for this study evaluating the feasibility of conducting a randomized control trial of a novel tri-compartment offloader brace for knee osteoarthritis. We involved patients as partners to enhance study design, implementation and interpretation of key outcomes.
Approach: Patient involvement consisted of two patient leaders and five patient advisors. Patients participated in 2 virtual focus groups to discuss study outcomes, protocol, results and knowledge translation. Patients were involved in all aspects of the research cycle.
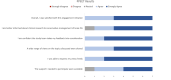
Outcomes: Patient feedback resulted in changes to study design, documentation, participant recruitment, data collection, results interpretation and knowledge dissemination, improving the participant experience and aligning study outcomes with patient priorities. Study participants showed a high level of protocol adherence and follow-up rates were excellent. We experienced several unexpected benefits including genuine friendships, a deeper understanding of the patient experience, a more pragmatic approach to clinical research, and leadership opportunities for patients.
Recommendations: We agreed on POR "non-negotiables" to ensure a positive experience for everyone, including creating a safe and comfortable environment, being genuinely receptive to patient feedback, and providing appropriate supports for patients. We strongly recommend that researchers (1) involve patients as early as possible, (2) provide ample and equal opportunities for all patients to be involved, and (3) address system hierarchy by involving patients as equals and fully considering all patient ideas from the beginning of the project.
Conclusions: While POR is a learning process that is often more challenging than the traditional clinical research approach, the benefits are well worth the additional time and effort required to do it well. Over time, our team experienced a cultural shift and evolution from a top-down research approach to a more inclusive approach considering patient voices as equal to those of researchers. Patient involvement in all aspects of the research process, from question development to results interpretation and dissemination is integral to clinical research advancing equitably.
Keywords: Clinical research; Feasibility study; Knee brace; Knee osteoarthritis; Patient partners; Patient-oriented research.
Plain language summary
Involving patients as partners in research can be challenging. However, the benefits are well worth the additional time and effort required to do it well. Patients improve the relevance, success and impact of clinical research, and are central to clinical research advancing. We partnered with seven patients from the community to conduct a research study assessing the effectiveness of a new knee brace for people with knee osteoarthritis. Patients participated in 2 virtual Zoom discussions and were involved in all aspects of the research process. The feedback received from patients led to many helpful changes to the study design, documents, recruitment strategies, data collection, and how to understand and share our results. These changes made the study more aligned with patient priorities and improved participants’ experience. Study participants followed study instructions and the majority completed the study. Our team also experienced many unexpected benefits of involving patients in research including genuine friendships, a deeper understanding of the patient experience, a more practical approach to clinical research, and more leadership opportunities for patients. We agreed that for everyone on the team to have a good experience, it’s important to create a safe and comfortable environment, really listen to and consider patient input, and provide patients the tools and trainings they need to fully participate. We recommend that researchers involve patients in their studies as early as possible, give patients lots of opportunities to be involved, and involve patients as equals, considering their ideas from start to finish.
© 2024. The Author(s).
Conflict of interest statement
ELB is supported by a Mitacs Accelerate Postdoctoral Fellowship on which the partner organization is Spring Loaded Technology. JLR receives research funding from Spring Loaded Technology. Spring Loaded Technology employees were not involved in the analysis, interpretation or reporting of data.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Patient Engagement Partnerships in Clinical Trials: Development of Patient Partner and Investigator Decision Aids.Patient. 2020 Dec;13(6):745-756. doi: 10.1007/s40271-020-00460-5. Epub 2020 Oct 7. Patient. 2020. PMID: 33026639 Free PMC article.
-
Enhancing patient-oriented research training: participant perceptions of an online course.Res Involv Engagem. 2024 Sep 6;10(1):93. doi: 10.1186/s40900-024-00629-4. Res Involv Engagem. 2024. PMID: 39242586 Free PMC article.
-
Benefits, barriers and recommendations for youth engagement in health research: combining evidence-based and youth perspectives.Res Involv Engagem. 2024 Sep 2;10(1):92. doi: 10.1186/s40900-024-00607-w. Res Involv Engagem. 2024. PMID: 39223602 Free PMC article. Review.
-
Protocol for co-producing a framework and integrated resource platform for engaging patients in laboratory-based research.Res Involv Engagem. 2024 Feb 12;10(1):25. doi: 10.1186/s40900-024-00545-7. Res Involv Engagem. 2024. PMID: 38347658 Free PMC article. Review.
References
-
- Canadian Institutes of Health Research. Strategy for Patient-Oriented Research. https://cihr-irsc.gc.ca/e/41204.html. Accessed on September 18, 2024.
-
- Duffett L. Patient engagement: What partnering with patient in research is all about. Thromb Res. Elsevier Ltd; 2017;150:113–20. 10.1016/j.thromres.2016.10.029 - PubMed
Publication types
Grants and funding
- IT13127/Mitacs
- IT13127/Mitacs
- 169387/CAPMC/ CIHR/Canada
- 169387/CAPMC/ CIHR/Canada
- 169387/CAPMC/ CIHR/Canada
- 169387/CAPMC/ CIHR/Canada
- 169387/CAPMC/ CIHR/Canada
- 169387/CAPMC/ CIHR/Canada
- Clinical Research Fund/Cumming School of Medicine
- Clinical Research Fund/Cumming School of Medicine
- Clinical Research Fund/Cumming School of Medicine
- Clinical Research Fund/Cumming School of Medicine
- Catalyst Grant/McCaig Institute for Bone and Joint Health
- Catalyst Grant/McCaig Institute for Bone and Joint Health
- Catalyst Grant/McCaig Institute for Bone and Joint Health
- Catalyst Grant/Bone and Joint Health Strategic Clinical Network
- Catalyst Grant/Bone and Joint Health Strategic Clinical Network
- Catalyst Grant/Bone and Joint Health Strategic Clinical Network
LinkOut - more resources
Full Text Sources

