Microgels With Electrostatically Controlled Molecular Affinity to Direct Morphogenesis
- PMID: 39449199
- PMCID: PMC11756038
- DOI: 10.1002/adma.202409731
Microgels With Electrostatically Controlled Molecular Affinity to Direct Morphogenesis
Abstract
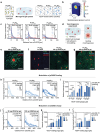
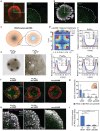
Concentration gradients of soluble signaling molecules-morphogens-determine the cellular organization in tissue development. Morphogen-releasing microgels have shown potential to recapitulate this principle in engineered tissue constructs, however, with limited control over the molecular cues in space and time. Inspired by the functionality of sulfated glycosaminoglycans (sGAGs) in morphogen signaling in vivo, a library of sGAG-based microgels is developed and designated as µGel Units to Instruct Development (µGUIDEs). Adjustment of the microgel's sGAG sulfation patterns and concentration enabled the programming of electrostatic affinities that control the release of morphogens. Based on computational analyses of molecular transport processes, µGUIDEs provided unprecedented precision in the spatiotemporal modulation of vascular endothelial growth factor (VEGF) gradients in a microgel-in-gel vasculogenesis model and kidney organoid cultures. The versatile approach offers new options for creating morphogen signaling centers to advance the understanding of tissue and organ development.
Keywords: VEGF; artificial signaling centers; beads; heparin; kidney organoids; microgels; morphogen gradients; sulfated glycosaminoglycans; vascular morphogenesis.
© 2024 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
U.F. and C.W. are co‐inventors of a patent (WO2010060485A1) covering the hydrogel materials used in this study. They also hold shares in the spin‐off company ZetaScience GmbH, Dresden, offering customized hydrogel precursors.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

