Exploring RNA therapeutics for urea cycle disorders
- PMID: 39449289
- PMCID: PMC11586603
- DOI: 10.1002/jimd.12807
Exploring RNA therapeutics for urea cycle disorders
Abstract
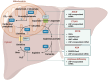
RNA has triggered a significant shift in modern medicine, providing a promising way to revolutionize disease treatment methods. Different therapeutic RNA modalities have shown promise to replace, supplement, correct, suppress, or eliminate the expression of a targeted gene. Currently, there are 22 RNA-based drugs approved for clinical use, including the COVID-19 mRNA vaccines, whose unprecedented worldwide success has meant a definitive boost in the RNA research field. Urea cycle disorders (UCD), liver diseases with high mortality and morbidity, may benefit from the progress achieved, as different genetic payloads have been successfully targeted to liver using viral vectors, N-acetylgalactosamine (GalNAc) conjugations or lipid nanoparticles (LNP). This review explores the potential of RNA-based medicines for UCD and the ongoing development of applications targeting specific gene defects, enzymes, or transporters taking part in the urea cycle. Notably, LNP-formulated mRNA therapy has been assayed preclinically for citrullinemia type I (CTLN1), adolescent and adult citrin deficiency, argininosuccinic aciduria, arginase deficiency and ornithine transcarbamylase deficiency, in the latter case has progressed to the clinical trials phase.
Keywords: antisense oligonucleotides; mRNA therapy; ornithine transcarbamylase deficiency; pseudoexon; splicing; urea cycle.
© 2024 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Eva Richard, Ainhoa Martinez‐Pizarro, and Lourdes R. Desviat declare they have no conflict of interest.
Figures
References
-
- Ah Mew N, Simpson KL, Gropman AL, et al. Urea cycle disorders overview. In: Adam MP, Feldman J, Mirzaa GM, et al., eds. GeneReviews®. University of Washington; 1993.
-
- Oude Blenke E, Schiffelers RM, Mastrobattista E. Oligonucleotides and mRNA therapeutics. In: Crommelin DJA, Sindelar RD, Meibohm B, eds. Pharmaceutical Biotechnology: Fundamentals and Applications. Springer International Publishing; 2024:291‐321.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

