Complete Remission with Inotuzumab Ozogamicin as Fourth-Line Salvage Therapy in a Child with Relapsed/Refractory Acute Lymphoblastic Leukemia
- PMID: 39449299
- PMCID: PMC11503335
- DOI: 10.3390/hematolrep16040056
Complete Remission with Inotuzumab Ozogamicin as Fourth-Line Salvage Therapy in a Child with Relapsed/Refractory Acute Lymphoblastic Leukemia
Abstract
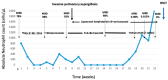
Background: Despite the progress achieved regarding survival rates in childhood acute lymphoblastic leukemia (ALL), relapsed or refractory disease still poses a therapeutic challenge. Inotuzumab ozogamicin is a CD22-directed monoclonal antibody conjugated to calicheamicin, which has been approved by the Food and Drug Administration for adults and pediatric patients 1 year and older with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia. Case presentation: Herein, we present the case of a 23-month-old girl with high-risk B-ALL who experienced very early isolated medullary relapse; following the failure of conventional chemotherapy according to the ALL-IC REL 2016 protocol, she went on to receive the bispecific T-cell engager (BiTE) blinatumomab and subsequently, due to refractory disease, the combination of fludarabine, cytarabine, and the proteasome inhibitor bortezomib without achieving remission. Given the high CD22 expression by the lymphoblasts, off-label use of inotuzumab ozogamicin (InO) was chosen and administrated in a 28-day cycle as a salvage treatment. The minimal residual disease (MRD) was 0.08% on day 28, and InO was continued, thus achieving MRD negativity; the patient successfully underwent an allogeneic stem cell transplantation from a matched family donor. Conclusions: Our case highlights the efficacy and safety of InO as a salvage treatment in the setting of relapsed B-ALL refractory not only to conventional chemotherapy but also to novel treatments, such as blinatumomab and bortezomib.
Keywords: acute lymphoblastic leukemia; childhood; inotuzumab ozogamicin.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- Hunger S.P., Lu X., Devidas M., Camitta B.M., Gaynon P.S., Winick N.J., Reaman G.H., Carroll W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. - DOI - PMC - PubMed
-
- Oskarsson T., Soderhall S., Arvidson J., Forestier E., Montgomery S., Bottai M., Lausen B., Carlsen N., Hellebostad M., Lähteenmäki P., et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: Prognostic factors, treatment and outcome. Haematologica. 2015;101:68–76. doi: 10.3324/haematol.2015.131680. - DOI - PMC - PubMed
-
- Rheingold S.R., Ji L., Xu X., Devidas M., Brown P.A., Gore L., Winick N.J., Carroll W.L., Hunger S., Raetz E.A., et al. Prognostic factors for survival after relapsed acute lymphoblastic leukemia (ALL): A Children’s Oncology Group (COG) study. J. Clin. Oncol. 2019;37:10008. doi: 10.1200/JCO.2019.37.15_suppl.10008. - DOI
-
- Dijoseph J., Armellino D., Boghaert E., Khandke K., Dougher M., Sridharan L., Kunz A., Hamann P.R., Gorovits B., Udata C., et al. Antibody-targeted chemotherapy with CMC-544: A CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103:1807–1814. doi: 10.1182/blood-2003-07-2466. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources