The Real-World Outcomes of Relapsed/Refractory Multiple Myeloma Treated with Elotuzumab, Pomalidomide, and Dexamethasone
- PMID: 39449301
- PMCID: PMC11503276
- DOI: 10.3390/hematolrep16040058
The Real-World Outcomes of Relapsed/Refractory Multiple Myeloma Treated with Elotuzumab, Pomalidomide, and Dexamethasone
Abstract
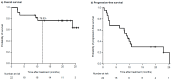
Introduction: A combination of elotuzumab, pomalidomide, and dexamethasone (EPd) was approved for the treatment of relapsed/refractory multiple myeloma (RRMM) following the ELOQUENT-3 phase II clinical trial. However, the clinical experience with this therapy is still limited. In this retrospective study, we analyzed the efficacy and safety of EPd in a real-world cohort of RRMM patients. Patients and Methods: The medical records of 22 patients who received EPd for RRMM at Yokohama Municipal Citizen's Hospital (Japan) between January 2020 and July 2021 were reviewed. Results: The median age of our cohort was 73.5 years. The overall response rate was 55%. With a median follow-up of 20.2 months, the median progression-free survival (PFS) was 9.1 months (95% confidence interval [CI], 2.5-23.0 months). The median PFS was shorter in patients with a poor performance status (PS) than in those with favorable PS (2.5 vs. 10.8 months; p < 0.01). Patients with prior daratumumab had significantly shorter PFS than those without prior daratumumab (2.1 vs. 23.0 months; p < 0.01). Additionally, patients with prior pomalidomide had significantly shorter PFS (1.7 vs. 10.3 months; p < 0.01). In the multivariate analysis, poor PS (hazard ratio [HR] = 4.1, 95% CI: 1.1-15.6; p = 0.04) and prior exposure to daratumumab (HR = 3.8, 95% CI: 1.1-13.8; p = 0.04) remained significantly associated with shorter PFS. Conclusions: The results of our study suggest that EPd is an active and well-tolerated regimen in RRMM, even in real-world patients. Furthermore, EPd may be useful, especially in daratumumab-naïve patients.
Keywords: elotuzumab; multiple myeloma; pomalidomide; relapse/refractory; transplant ineligible.
Conflict of interest statement
The authors have no relevant financial or nonfinancial interests to disclose.
Figures

Similar articles
-
Elotuzumab Plus Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma: Final Overall Survival Analysis From the Randomized Phase II ELOQUENT-3 Trial.J Clin Oncol. 2023 Jan 20;41(3):568-578. doi: 10.1200/JCO.21.02815. Epub 2022 Aug 12. J Clin Oncol. 2023. PMID: 35960908 Free PMC article. Clinical Trial.
-
Daratumumab as Single Agent in Relapsed/Refractory Myeloma Patients: A Retrospective Real-Life Survey.Front Oncol. 2021 Mar 5;11:624405. doi: 10.3389/fonc.2021.624405. eCollection 2021. Front Oncol. 2021. PMID: 33763359 Free PMC article.
-
Elotuzumab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma: a multicenter, retrospective, real-world experience with 200 cases outside of controlled clinical trials.Haematologica. 2024 Jan 1;109(1):245-255. doi: 10.3324/haematol.2023.283251. Haematologica. 2024. PMID: 37439329 Free PMC article.
-
Daratumumab for the Management of Newly Diagnosed and Relapsed/Refractory Multiple Myeloma: Current and Emerging Treatments.Front Oncol. 2021 Feb 17;10:624661. doi: 10.3389/fonc.2020.624661. eCollection 2020. Front Oncol. 2021. PMID: 33680948 Free PMC article. Review.
-
Immunotherapy: A New Approach to Treating Multiple Myeloma with Daratumumab and Elotuzumab.Ann Pharmacother. 2016 Jul;50(7):555-68. doi: 10.1177/1060028016642786. Epub 2016 Apr 15. Ann Pharmacother. 2016. PMID: 27083916 Free PMC article. Review.
Cited by
-
Revolutions at the frontline of multiple myeloma treatment: lessons and challenges to finding a cure.Front Oncol. 2025 Jun 20;15:1578529. doi: 10.3389/fonc.2025.1578529. eCollection 2025. Front Oncol. 2025. PMID: 40626006 Free PMC article. Review.
References
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
-
- Kumar S.K., Dispenzieri A., Lacy M.Q., Gertz M.A., Buadi F.K., Pandey S., Kapoor P., Dingli D., Hayman S.R., Leung N., et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. doi: 10.1038/leu.2013.313. - DOI - PMC - PubMed
-
- Sonneveld P., Goldschmidt H., Rosinol L., Blade J., Lahuerta J.J., Cavo M., Tacchetti P., Zamagni E., Attal M., Lokhorst H.M., et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: A meta-analysis of phase III randomized, controlled trials. J. Clin. Oncol. 2013;31:3279–3287. doi: 10.1200/JCO.2012.48.4626. - DOI - PubMed
-
- Gay F., Larocca A., Wijermans P., Cavallo F., Rossi D., Schaafsma R., Genuardi M., Romano A., Liberati A.M., Siniscalchi A., et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: Analysis of 1175 patients. Blood. 2011;117:3025–3031. doi: 10.1182/blood-2010-09-307645. - DOI - PubMed
LinkOut - more resources
Full Text Sources

