Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Adult Patients with Acute Lymphoblastic Leukemia: Results of a Single-Center Study
- PMID: 39449305
- PMCID: PMC11503301
- DOI: 10.3390/hematolrep16040062
Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Adult Patients with Acute Lymphoblastic Leukemia: Results of a Single-Center Study
Abstract
Background: Despite the adoption of pediatric-like chemotherapy protocols, the introduction of new immunotherapies and a better understanding of the oncogenic landscape, the outcome for adult patients with acute lymphoblastic leukemia (ALL) remain substantially dismal. The aim of the present study was to evaluate the outcome in terms of survival in a cohort of adult patients with ALL who received allogeneic hematopoietic stem cell transplantation (alloSCT) between 2013 and 2023.
Methods: This was a single-center observational retrospective study including all consecutive adult patients with ALL who received an alloSCT between April 2013 and April 2023 at the Stem Cell Transplant Center AOU Città della Salute e della Scienza of Torino. The primary endpoints were overall survival (OS), graft-versus-host disease (GVHD) Relapse-Free Survival (GRFS), Leukemia-Free Survival (LFS) and cumulative incidence (CI) of Non-Relapse Mortality (NRM).
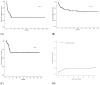
Results: The 4-year OS and LFS were 63.4% and 48.1%, respectively, and the 1-year GRFS was 42.9%. The 1-year CI of bloodstream infections (BSI), invasive fungal infections and NRM were 38%, 7% and 18.4%, respectively. Multivariate analysis showed that the use of total body irradiation (TBI), a time interval from diagnosis to alloSCT 7 months and female gender were factors significantly associated with better OS. Relapse of the underlying malignancy and BSI were the main causes of death.
Conclusion: Our study suggests that alloSCT from a matched sibling donor (MSD) and alternative donors may be considered an effective tool for patients with ALL achieving a CR.
Keywords: acute lymphoblastic leukemia; allogeneic stem cell transplantation; total body irradiation.
Conflict of interest statement
The authors have stated that they have no conflicts of interest.
Figures
References
-
- Gaynon P.S., Angiolillo A.L., Carroll W.L., Nachman J.B., Trigg M.E., Sather H.N., Hunger S.P., Devidas M., Children’s Oncology Group Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: A Children’s Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. - DOI - PMC - PubMed
-
- Pulte D., Jansen L., Gondos A., Katalinic A., Barnes B., Ressing M., Holleczek B., Eberle A., Brenner H., GEKID Cancer Survival Working Group Survival of adults with acute lymphoblastic leukemia in Germany and the United States. PLoS ONE. 2014;9:e85554. doi: 10.1371/journal.pone.0085554. - DOI - PMC - PubMed
-
- Kantarjian H., Thomas D., O’Brien S., Cortes J., Giles F., Jeha S., Bueso-Ramos C.E., Pierce S., Shan J., Koller C., et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous