How the Oocyte Nucleolus Is Turned into a Karyosphere: The Role of Heterochromatin and Structural Proteins
- PMID: 39449320
- PMCID: PMC11503394
- DOI: 10.3390/jdb12040028
How the Oocyte Nucleolus Is Turned into a Karyosphere: The Role of Heterochromatin and Structural Proteins
Abstract
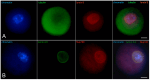
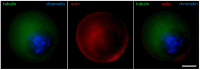
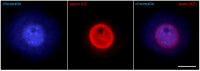
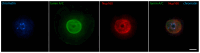
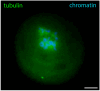
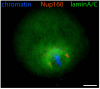
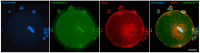
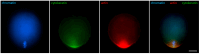
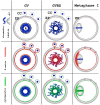
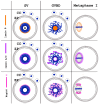
Oocyte meiotic maturation includes large-scale chromatin remodeling as well as cytoskeleton and nuclear envelope rearrangements. This review addresses the dynamics of key cytoskeletal proteins (tubulin, actin, vimentin, and cytokeratins) and nuclear envelope proteins (lamin A/C, lamin B, and the nucleoporin Nup160) in parallel with chromatin reorganization in maturing mouse oocytes. A major feature of this reorganization is the concentration of heterochromatin into a spherical perinucleolar rim called surrounded nucleolus or karyosphere. In early germinal vesicle (GV) oocytes with non-surrounded nucleolus (without karyosphere), lamins and Nup160 are at the nuclear envelope while cytoplasmic cytoskeletal proteins are outside the nucleus. At the beginning of karyosphere formation, lamins and Nup160 follow the heterochromatin relocation assembling a new spherical structure in the GV. In late GV oocytes with surrounded nucleolus (fully formed karyosphere), the nuclear envelope gradually loses its integrity and cytoplasmic cytoskeletal proteins enter the nucleus. At germinal vesicle breakdown, lamin B occupies the karyosphere interior while all the other proteins stay at the karyosphere border or connect to chromatin. In metaphase oocytes, lamin A/C surrounds the spindle, Nup160 localizes to its poles, actin and lamin B are attached to the spindle fibers, and cytoplasmic intermediate filaments associate with both the spindle fibers and the metaphase chromosomes.
Keywords: cytoskeleton; germinal vesicle; karyosphere; meiosis; meiotic spindle; nuclear envelope; nuclear lamina; oocytes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

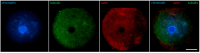

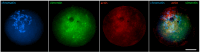
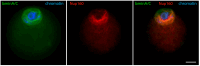
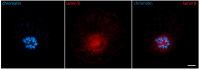

Similar articles
-
Dynamics of Lamins B and A/C and Nucleoporin Nup160 during Meiotic Maturation in Mouse Oocytes.Folia Biol (Praha). 2017;63(1):6-12. doi: 10.14712/fb2017063010006. Folia Biol (Praha). 2017. PMID: 28374669
-
Special Nuclear Structures in the Germinal Vesicle of the Common Frog with Emphasis on the So-Called Karyosphere Capsule.J Dev Biol. 2023 Dec 12;11(4):44. doi: 10.3390/jdb11040044. J Dev Biol. 2023. PMID: 38132712 Free PMC article.
-
Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function.Dev Biol. 2004 Nov 15;275(2):447-58. doi: 10.1016/j.ydbio.2004.08.028. Dev Biol. 2004. PMID: 15501230
-
Chromatin Configuration in Diplotene Mouse and Human Oocytes during the Period of Transcriptional Activity Extinction.Int J Mol Sci. 2023 Jul 15;24(14):11517. doi: 10.3390/ijms241411517. Int J Mol Sci. 2023. PMID: 37511273 Free PMC article. Review.
-
Karyosphere (Karyosome): A Peculiar Structure of the Oocyte Nucleus.Int Rev Cell Mol Biol. 2018;337:1-48. doi: 10.1016/bs.ircmb.2017.12.001. Epub 2018 Jan 12. Int Rev Cell Mol Biol. 2018. PMID: 29551157 Review.
References
-
- Choi T., Rulong S., Resau J., Fukasawa K., Matten W., Kuriyama R., Mansour S., Ahn N., Vande Woude G.F. Mos/mitogen-activated protein kinase can induce early meiotic phenotypes in the absence of maturation-promoting factor: A novel system for analyzing spindle formation during meiosis I. Proc. Natl. Acad. Sci. USA. 1996;93:4730–4735. doi: 10.1073/pnas.93.10.4730. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

