Development of a Mammalian Cell Line for Stable Production of Anti-PD-1
- PMID: 39449324
- PMCID: PMC11503334
- DOI: 10.3390/antib13040082
Development of a Mammalian Cell Line for Stable Production of Anti-PD-1
Abstract
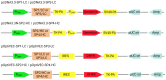
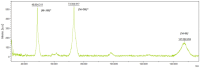
Background/Objectives: Immune checkpoint blockade, particularly targeting the programmed cell death 1 (PD-1) receptor, is a promising strategy in cancer immunotherapy. The interaction between PD-1 and its ligands, PD-L1 and PD-L2, is crucial in immune evasion by tumors. Blocking this interaction with monoclonal antibodies like Nivolumab can restore anti-tumor immunity. This study aims to develop a stable expression system for Nivolumab-based anti-PD-1 in the Chinese Hamster Ovary (CHO) DG44 cell line using two different expression vector systems with various signal sequences. Methods: The heavy chain (HC) and light chain (LC) of Nivolumab were cloned into two expression vectors, pOptiVEC and pcDNA3.3. Each vector was engineered with two distinct signal sequences, resulting in the creation of eight recombinant plasmids. These plasmids were co-transfected into CHO DG44 cells in different combinations, allowing for the assessment of stable antibody production. Results: Both pOptiVEC and pcDNA3.3 vectors were successful in stably integrating and expressing the Nivolumab-based anti-PD-1 antibody in CHO DG44 cells. This study found that the choice of signal sequence significantly influenced the quantity of antibodies produced. The optimization of production conditions further enhanced antibody yield, indicating the potential for large-scale production. Conclusions: This study demonstrates that both pOptiVEC and pcDNA3.3 expression systems are effective for the stable production of Nivolumab-based anti-PD-1 in CHO DG44 cells. Signal sequences play a critical role in determining the expression levels, and optimizing production conditions can further increase antibody yield, supporting future applications in cancer immunotherapy.
Keywords: CHO DG44; Nivolumab; anti-PD-1; immune checkpoint molecule; monoclonal antibody production.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates.Cancer Immunol Res. 2014 Sep;2(9):846-56. doi: 10.1158/2326-6066.CIR-14-0040. Epub 2014 May 28. Cancer Immunol Res. 2014. PMID: 24872026
-
[Expression of human IL-35-IgG4 (Fc) fusion protein in CHO/DG44 cells].Sheng Wu Gong Cheng Xue Bao. 2009 Jan;25(1):109-15. Sheng Wu Gong Cheng Xue Bao. 2009. PMID: 19441235 Chinese.
-
On the optimal ratio of heavy to light chain genes for efficient recombinant antibody production by CHO cells.Biotechnol Prog. 2005 Jan-Feb;21(1):122-33. doi: 10.1021/bp049780w. Biotechnol Prog. 2005. PMID: 15903249
-
Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature.J Immunother Cancer. 2018 Nov 20;6(1):126. doi: 10.1186/s40425-018-0439-2. J Immunother Cancer. 2018. PMID: 30458852 Free PMC article. Review.
Cited by
-
Construction of an Integration Vector with a Chimeric Signal Peptide for the Expression of Monoclonal Antibodies in Mammalian Cells.Curr Issues Mol Biol. 2024 Dec 22;46(12):14464-14475. doi: 10.3390/cimb46120868. Curr Issues Mol Biol. 2024. PMID: 39727996 Free PMC article.
References
-
- Barentine C. Increasing Production of Therapeutic mAbs in CHO Cells through Genetic Engineering. Utah State University; Logan, UT, USA: 2022.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

