A Novel Newborn Screening Program for Sickle Cell Disease in Nigeria
- PMID: 39449355
- PMCID: PMC11503303
- DOI: 10.3390/ijns10040067
A Novel Newborn Screening Program for Sickle Cell Disease in Nigeria
Abstract
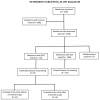
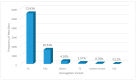
Newborn screening for sickle cell disease (SCD) is sparse in sub-Saharan Africa. The leadership of the Aminu Kano Teaching Hospital (AKTH) in Kano, Nigeria, with the support of local religious authorities, established a groundbreaking SCD newborn screening program that has become the standard of care for pregnant women and their newborns. Our program includes (1) prenatal genetic counseling for all pregnant women in the antenatal clinic, (2) newborn screening, (3) postnatal genetic counseling for parents of newborns diagnosed with SCD and SCT, and (4) referral of newborns with SCD for follow-up in the SCD Comprehensive Care Clinic by 3 months of age. From September 2020 to December 2023, the team screened 7530 infants for SCD at the AKTH, identifying 126 (1.7%) infants with SCD and 1546 (20.5%) with SCT. Of these, 93 (73.8%) newborns with SCD received individualized genetic counseling, and 43 (46%) were referred to the SCD Comprehensive Care Clinic before 3 months. Group genetic counseling was provided to the parents of 778 (50.3%) of newborns identified with SCT. The SCD newborn screening at the AKTH is now standard care, indicating the viability of sustaining an SCD newborn screening program that provides pre- and postnatal genetic counseling and comprehensive SCD care within a low-income setting.
Keywords: Africa; genetic counseling; newborn screening; sickle cell disease.
Conflict of interest statement
M.R. DeBaun and his institution sponsored two externally funded research investigator-initiated projects. Global Blood Therapeutics (GBT) provided funding for the cost of the clinical studies. GBT was not a co-sponsor of either study. DeBaun did not receive any compensation for conducting these two investigator-initiated observational studies. DeBaun was a medical advisor in developing the CTX001 Early Economic Model. DeBaun provided medical input on the economic model as part of an expert reference group for the Vertex/CRISPR CTX001 Early Economic Model in 2020. DeBaun consulted for the Forma Pharmaceutical company about sickle cell disease in 2021 and 2022. DeBaun is on the chair of the steering committee for a Novartis-sponsored phase II trial to prevent priapism in men with sickle cell disease. Y. Carroll received funding for three educational grants. Y. Carroll was the co-PI on two ACCELL grants from the Global Blood Therapeutics (GBT) Foundation to train nurses on SCD in 2021 and 2022. Additionally, Y. Carroll is co-PI on a US Department of Agriculture Grant 2024–2025 to train healthcare professionals on SCD. Y. Carroll did not receive any compensation for these grants. The remaining authors declare no conflicts of interest.
Figures
References
-
- Angastiniotis M., Modell B., Englezos P., Boulyjenkov V. Prevention and control of haemoglobinopathies. [(accessed on 17 September 2024)];Bull. World Health Organ. 1995 73:375. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2486673/ - PMC - PubMed
-
- Saraf S.L., Molokie R.E., Nouraie M., Sable C.A., Luchtman-Jones L., Ensing G.J., Campbell A.D., Rana S.R., Niu X.M., Machado R.F., et al. Differences in the clinical and genotypic presentation of sickle cell disease around the world. Paediatr. Respir. Rev. 2014;15:4–12. doi: 10.1016/j.prrv.2013.11.003. - DOI - PMC - PubMed
-
- Nnodu O.E., Sopekan A., Nnebe-Agumadu U., Ohiaeri C., Adeniran A., Shedul G., Isa H.A., Owolabi O., Chianumba R.I., Tanko Y., et al. Implementing newborn screening for sickle cell disease as part of immunization programs in Nigeria: A feasibility study. Lancet Haematol. 2020;7:e534–e540. doi: 10.1016/S2352-3026(20)30143-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

