Platelet Extracellular Vesicles Loaded Gelatine Hydrogels for Wound Care
- PMID: 39449544
- PMCID: PMC11694086
- DOI: 10.1002/adhm.202401914
Platelet Extracellular Vesicles Loaded Gelatine Hydrogels for Wound Care
Abstract
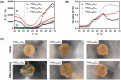
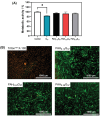
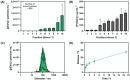
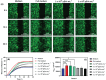
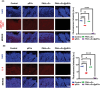
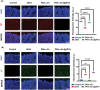
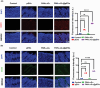
Platelet extracellular vesicles (pEVs) isolated from clinical-grade human platelet concentrates are attracting attention as a promising agent for wound healing therapies. Although pEVs have shown potential for skin regeneration, their incorporation into wound bandages has remained limitedly explored. Herein, gelatine-based hydrogel (PAH-G) foams for pEVs loading and release are formulated by crosslinking gelatine with poly(allylamine) hydrochloride (PAH) in the presence of glutaraldehyde and sodium bicarbonate. The optimized PAH-G hydrogel foam, PAH0.24G37, displayed an elastic modulus G' = 8.5 kPa at 37 °C and retained a rubbery state at elevated temperatures. The excellent swelling properties of PAH0.24G37 allowed to easily absorb pEVs at high concentration (1 × 1011 particles mL-1). The therapeutic effect of pEVs was evaluated in vivo on a chronic wound rat model. These studies demonstrated full wound closure after 14 days upon treatment with PAH0.24G37@pEVs. The maintenance of a reduced-inflammatory environment from the onset of treatment promoted a quicker transition to skin remodeling. Promotion of follicle activation and angiogenesis as well as M1-M2 macrophage modulation are evidenced. Altogether, the multifunctional properties of PAH0.24G37@pEVs addressed the complex challenges associated with chronic diabetic wounds, representing a significant advance toward personalized treatment regimens for these conditions.
Keywords: chronic wounds; hydrogel foam; platelet extracellular vesicles; skin regeneration; wound model.
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Maheshwari G., Wounds APAC 2024, 7, 1–11, https://woundsasia.com/journal-articles/chronic-wounds-a-rising-public-h...;
- b) Eriksson E., Liu P. Y., Schultz G. S., Martins‐Green M. M., Tanaka R., Weir D., Gould L. J., Armstrong D. G., Gibbons G. W., Wolcott R., Olutoye O. O., Kirsner R. S., Gurtner G. C., Wound Rep. Reg. 2022, 30, 156. - PMC - PubMed
-
- Hugo A., Rodrigues T., Mader J. K., Knoll W., Bouchiat V., Boukherroub R., Szunerits S., Biosen. Bioelecton. X 2023, 13, 100305.
MeSH terms
Substances
Grants and funding
- ANR-21-ENM3-0002/French Renatech network, Belgian F.R.S - FNRS: R.8008.21, EuroNanomed III-GSkin
- 112-2622-E-038-006/National Science and Technology Council (NSTC)
- 112-2221-E-038-002-MY3/National Science and Technology Council (NSTC)
- 113-2923-E-038 -001-/National Science and Technology Council (NSTC)
LinkOut - more resources
Full Text Sources
Other Literature Sources

