Driving tert-butyl axial: the surprising cyclopropyl effect
- PMID: 39449689
- PMCID: PMC11494268
- DOI: 10.1039/d4sc05470a
Driving tert-butyl axial: the surprising cyclopropyl effect
Abstract
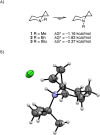
The presence of a small spirocyclic ring at an adjacent position alters the conformational preference for equatorial substitution in six-membered rings. DFT calculations and low-temperature 1H NMR experiments demonstrate that alkyl groups larger than methyl possess negative A-values when geminal to a spirocyclopropane, with larger groups such as isopropyl and tert-butyl being exclusively axial at -78 °C. Similar effects are found for heteroatoms, including halogens, and for a range of other electron-withdrawing substituents. Similar effects are observed for other strained rings (epoxide, cyclobutane, oxetane) and the concepts extend to acyclic models as well as heterocycles such as piperidines and piperazines. The origin of the effect is traced to an increase in torsional strain in combination with hyperconjugative effects in the case of electron-poor groups.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
LinkOut - more resources
Full Text Sources

