Optimization and validation of multi-echo, multi-contrast SAGE acquisition in fMRI
- PMID: 39449748
- PMCID: PMC11497078
- DOI: 10.1162/imag_a_00217
Optimization and validation of multi-echo, multi-contrast SAGE acquisition in fMRI
Abstract
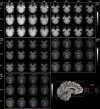
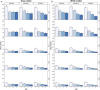
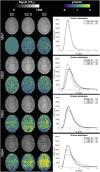
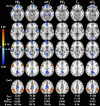
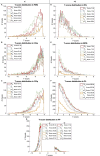
The purpose of this study was to optimize and validate a multi-contrast, multi-echo fMRI method using a combined spin- and gradient-echo (SAGE) acquisition. It was hypothesized that SAGE-based blood oxygen level-dependent (BOLD) functional MRI (fMRI) will improve sensitivity and spatial specificity while reducing signal dropout. SAGE-fMRI data were acquired with five echoes (2 gradient-echoes, 2 asymmetric spin-echoes, and 1 spin-echo) across 12 protocols with varying acceleration factors, and temporal SNR (tSNR) was assessed. The optimized protocol was then implemented in working memory and vision tasks in 15 healthy subjects. Task-based analysis was performed using individual echoes, quantitative dynamic relaxation times T2 * and T2, and echo time-dependent weighted combinations of dynamic signals. These methods were compared to determine the optimal analysis method for SAGE-fMRI. Implementation of a multiband factor of 2 and sensitivity encoding (SENSE) factor of 2.5 yielded adequate spatiotemporal resolution while minimizing artifacts and loss in tSNR. Higher BOLD contrast-to-noise ratio (CNR) and tSNR were observed for SAGE-fMRI relative to single-echo fMRI, especially in regions with large susceptibility effects and for T2-dominant analyses. Using a working memory task, the extent of activation was highest with T2 *-weighting, while smaller clusters were observed with quantitative T2 * and T2. SAGE-fMRI couples the high BOLD sensitivity from multi-gradient-echo acquisitions with improved spatial localization from spin-echo acquisitions, providing two contrasts for analysis. SAGE-fMRI provides substantial advantages, including improving CNR and tSNR for more accurate analysis.
Keywords: BOLD contrast; BOLD contrast-to-noise ratio (CNR); functional MRI (fMRI); multi-echo fMRI; spin-echo fMRI.
© 2024 Massachusetts Institute of Technology. Published under a Creative Commons Attribution 4.0 International (CC BY 4.0) license.
Conflict of interest statement
Dr. Stokes has received speaking fees from Eli Lilly.
Figures




References
-
- Barch , D. M. , Burgess , G. C. , Harms , M. P. , Petersen , S. E. , Schlaggar , B. L. , Corbetta , M. , Glasser , M. F. , Curtiss , S. , Dixit , S. , Feldt , C. , Nolan , D. , Bryant , E. , Hartley , T. , Footer , O. , Bjork , J. M. , Poldrack , R. , Smith , S. , Johansen-Berg , H. , Snyder , A. Z. , & Van Essen , D. C. ( 2013. ). Function in the human connectome: Task-fMRI and individual differences in behavior . NeuroImage , 80 , 169 – 189 . 10.1016/J.NEUROIMAGE.2013.05.033 - DOI - PMC - PubMed
-
- Bell , L. C. , Does , M. D. , Stokes , A. M. , Baxter , L. C. , Schmainda , K. M. , Dueck , A. C. , & Quarles , C. C . ( 2017. ). Optimization of DSC MRI echo times for CBV measurements using error analysis in a pilot study of high-grade gliomas . AJNR American Journal of Neuroradiology , 38 ( 9 ), 1710 – 1715 . 10.3174/ajnr.A5295 - DOI - PMC - PubMed
-
- Binney , R. J. , Embleton , K. V. , Jefferies , E. , Parker , G. J. M. , & Ralph Lambon , A. M. ( 2010. ). The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: Evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia . Cerebral Cortex , 20 ( 11 ), 2728 – 2738 . 10.1093/cercor/bhq019 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
